2024Activity reportProject-TeamSAIRPICO
RNSR: 202324398Z- Research center Inria Centre at Rennes University
- In partnership with:INSERM, Institut Curie
- Team name: Space-time imaging, artificial intelligence and computing for cellular and chemical biology
- In collaboration with:Chimie et Biologie du Cancer
- Domain:Digital Health, Biology and Earth
- Theme:Computational Biology
Keywords
Computer Science and Digital Science
- A3.1.1. Modeling, representation
- A3.3. Data and knowledge analysis
- A3.3.3. Big data analysis
- A3.4. Machine learning and statistics
- A3.4.1. Supervised learning
- A3.4.5. Bayesian methods
- A3.4.6. Neural networks
- A3.4.7. Kernel methods
- A3.4.8. Deep learning
- A5.3. Image processing and analysis
- A5.3.2. Sparse modeling and image representation
- A5.3.3. Pattern recognition
- A5.3.4. Registration
- A5.4.1. Object recognition
- A5.4.4. 3D and spatio-temporal reconstruction
- A5.4.5. Object tracking and motion analysis
- A5.4.6. Object localization
- A5.9.1. Sampling, acquisition
- A5.9.2. Estimation, modeling
- A5.9.3. Reconstruction, enhancement
- A5.9.5. Sparsity-aware processing
- A5.9.6. Optimization tools
- A6.1.2. Stochastic Modeling
- A6.1.3. Discrete Modeling (multi-agent, people centered)
- A6.1.4. Multiscale modeling
- A6.1.5. Multiphysics modeling
- A6.2.3. Probabilistic methods
- A6.2.4. Statistical methods
- A6.2.6. Optimization
- A6.3. Computation-data interaction
- A6.3.1. Inverse problems
- A6.3.2. Data assimilation
- A6.3.3. Data processing
- A6.3.4. Model reduction
- A6.3.5. Uncertainty Quantification
- A9.2. Machine learning
- A9.3. Signal analysis
Other Research Topics and Application Domains
- B1.1.1. Structural biology
- B1.1.7. Bioinformatics
- B1.1.8. Mathematical biology
- B2.2.3. Cancer
- B2.6. Biological and medical imaging
1 Team members, visitors, external collaborators
Research Scientists
- Charles Kervrann [Team leader, INRIA, Senior Researcher]
- Anais Badoual [INRIA, Researcher, 0.8 ETP]
- Patrick Bouthemy [INRIA, Emeritus, from Nov 2024]
- Patrick Bouthemy [INRIA, Senior Researcher, until Nov 2024]
- Ludger Johannes [INSERM, Senior Researcher, INSERM-U1143, Institut Curie, Paris, 0.25 ETP]
- Massiullah Shafaq-Zadah [INSERM, Researcher, INSERM-U1143, Institut Curie, Paris, 0.25 ETP]
- Christian Wunder [INSERM, Researcher, INSERM-U1143, Institut Curie, Paris, 0.25 ETP]
Post-Doctoral Fellow
- Ilyes Hamitouche [INSERM, Post-Doctoral Fellow, until Sep 2024, INSERM-U1143, Institut Curie, Paris, 0.75 ETP]
PhD Students
- Lisa Balsollier [CNRS, until Sep 2024]
- Chencheng Gu [INRIA, from Mar 2024]
- Leo Maury [INRIA, from Jan 2024]
- Mounir Messaoudi [INRIA, from May 2024]
- Ferdinand Plesse–Costa [INRIA, from Nov 2024]
- Quentin Rapilly [INRIA]
- Quentin Tallon [IRSN, until Sep 2024]
Technical Staff
- Vincent Briane [INRIA (CDD), Engineer, until Feb 2024]
- Estelle Dransart [Institut Curie (IR, permanent position), Engineer, INSERM-U1143, Institut Curie, Paris, 0.25 ETP]
- Arthur Masson [INRIA (IR, permanent position), Engineer]
- Caio Vaz Rimoli [INSERM (IR, permanent position), Engineer, from Apr 2024, INSERM-U1143, Institut Curie, Paris, 0.75 ETP ]
Interns and Apprentices
- Theotim Barbier [INRIA, Intern, from Jul 2024 until Aug 2024]
- Ferdinand Plesse–Costa [INRIA, Intern, from Mar 2024 until Aug 2024]
- Nampoina Ravelomanana [INRIA, Intern, from Apr 2024 until Sep 2024]
Administrative Assistant
- Caroline Tanguy [INRIA]
External Collaborator
- Frédéric Lavancier [ENSAI , from Sep 2024]
2 Overall objectives
During the past two decades many ground-breaking technologies emerged and allowed the visualization of tissues, cells, proteins, viruses, and macromolecular structures at all levels of spatial resolution (from 10 nm to 150 nm). The discovery of fluorescent labeling probes (Green fluorescence Protein, Nobel Prize in chemistry 2008) and recent advances in optics and digital sensors (e.g., PALM, STED and SIM) have been key developments which have served to overcome the theoretical optical diffraction limit (200 nm) established in the 19th century. Because of these technological breakthroughs and their impacts in life sciences, contemporary microscopy has been praised through prestigious awards, such as the Nobel Prizes awarded to inventors of the concepts of super-resolution microscopy (2014) and cryo-electron microscopy (2017). Fluorescent microscopy imaging has become the spearhead of modern biology as it is able to generate videos comprising dozens of Gigabytes of data within an hour, and can depict long-term 4D nanoscale cell behaviors with low photo-toxicity. The ability to follow nanoscale cellular events is also proving to be of immense clinical relevance, especially for the study of cancer progression and viral infections. All these technological advances in microscopy have created new challenges for researchers in signal-image processing, and have even modified conventional paradigms once digital processing became a key component in the surmounting of the diffraction barrier (e.g., PALM and SIM).
All fluorescence microscopy systems record fluorescent signals emitted by molecules tagged with genetically engineered or chemically coupled proteins within cells. In a conventional setup photons are collected and registered at a given pixel (or voxel in 3D imaging). The measured fluorescence intensity is a scalar value, generally proportional to the density of tagged-molecules representing a few dozens of nanometers within a pixel/voxel. However, fluorescence necessarily includes intensity (biomolecule density), wavelength (absorption and emission spectrum), time (fluorescence decay lifetime) and polarization (which arises from the dipole orientation). Nevertheless, it is worth noting that the orientation of dipoles cannot be measured by conventional fluorescence microscopy setups. The next generation technology will be able to provide the missing directional information which is required to better reveal the structure and function of biomolecules and organelles in cells. Among the recent progress, let us mention polarized microscopy that has the potential to probe the dipole orientation of fluorophores linked to proteins or lipids of interest and thereby, to report valuable information about the orientation and diffusive behavior of the molecule. Light polarization technology is also very flexible since it can be advantageously combined with super-resolution microscopy to characterize the nanometric structural organization of filamentous assemblies (actin filaments, microtubules), of membrane lipid orientations or the global architecture of local assembly of both proteins and lipids. Given their promising potential in terms of flexibility and production of information at high spatial resolution in vivo, polarized microscopy vector-valued images are likely to be in the future as common as confocal scalar-valued images.
As the resulting image data are 3D+time multi-valued signals, potentially depicting several fluorescently tagged molecular species, the analysis and the interpretation of these signals represents a new challenge in signal image processing and statistical machine learning, and one for which several scientific barriers must be overcome. A first barrier is to reduce the high level of noise and blur observed in 3D+time vector-valued data, which encompass information about density and orientation of biomolecules. As the processing of very large temporal series of images considerably slows down the analysis, special attention must be paid to the feasibility and scalability of the developed algorithms. A second barrier is the interpretation of dynamic and structural information content of such vector-valued images, for which no general method currently exists. A third barrier relates to the possibility of producing 3D spatial high-resolution maps of molecular motions from data generated by conventional polarized microscopy instruments. These barriers translate into unsolved digital challenges which need to be surmounted in order for this technology to be adopted in large-scale biological studies.
As the current methods are limited in handling polarized images, SAIRPICO aims to create the next generation of information processing techniques required to overcome the aforementioned barriers, and to solve challenging image processing problems induced by the acquisition of 3D+time vector-valued images. The resulting algorithms will serve to characterize the dynamics of biomolecules (e.g., proteins, lipids, …) and to decipher the molecular transport pathways or the motion (e.g., migration) and deformation of cells, which is of considerable of interest in fundamental cell biology and for precision medicine.
3 Research program
Four complementary Research Axes will be investigated with scientists who develop chemical methods (e.g. advanced imaging probes such as non-natural clickable amino acids, linker chemistry) to improve the rigidity of linkers and the photo-stability of fluorophores required for robust estimation of orientation of single molecules and components of cytosolic machinery, as well as single-molecule FRET techniques to infer and quantify interactions between membrane proteins.
Methodological Research Axis 1 - Modeling and reconstruction of multi-valued images.
Development of cutting-edge computational strategies and mathematical frameworks for reconstructing multi-valued images. Structure-based sparse representations of multi-value images will be established from the analysis of the spatiotemporal correlations and the inherent redundancy of data in multiple images. We will investigate statistical nonparametric methods and aggregation techniques, variational Bayesian methods, including shape-based models, as well as machine learning strategies to solve the underlying inverse problems.
Methodological Research Axis 2 - Methods for high-resolution spatial quantification of molecular mobility and interactions.
Characterization of molecular mobility at the nanoscale from multi-valued images. We intend to fully exploit the rich contents of microscopy images in order to build single-molecule (e.g., endocytic ligands) and biomolecule (e.g., cytosolic machinery, metabolic sensors) tracking algorithms, derive robust estimators of molecular mobility, and quantify spatially-variable interactions between molecular species and cytoskeleton. The resulting algorithms will be used to produce high-resolution spatial maps of molecular mobility given stochastic motion models and sparse representations.
Methodological Research Axis 3 - Spatiotemporal modeling of 3D shapes, motions and deformations.
Development of shape models and descriptors to capture 3D motion and deformation of macromolecular complexes (cryo-electron tomography (cryo-ET), single particle analysis (SPA)) on one hand, and on the other hand, intracellular components and tumor cells, at the scale of a single cell and tissues. We intend to represent 3D shapes by parametric surfaces controlled by key points and to segment and track structures in 3D microscopy. The main originality will be to exploit annotations and/or high-level priors to derive features for classifying molecular conformations in cryo-ET, and phenotypes induced by drugs (single cell), or controlled hypoxia conditions (tissue scale) in 3D+time fluorescence microscopy.
Transversal Research Axis 4 - Analysis of case-studies in cell biology and cancer research.
Demonstration that the methods and algorithms related to the three previous methodological axes allow one to perform image reconstruction for several 3D instruments (TIRFM, Lattice Light Sheet Microscopy, Multi-Focus Microscopy, cryo-ET), and accurately quantify the shape and motion of cell components and biomolecules that interact with membranes and the cytoskeleton. The resulting images and features will be helpful to better decipher the intracellular dynamics of trafficking and signaling events in living cells, especially membrane mechanics at the cell surface, endocytosis, as well as signal transduction to the nucleus. The methods will be developed for investigation in cellular and chemical biology, and extended further to perform analysis at the tissue scale.
4 Application domains
The advances in SAIRPICO will result in a new generation of algorithms for multi-valued microscopy instruments, which will be widely used in the future in fundamental and applied cellular and chemical biology. The team gathers researchers developing new imaging modality and computational methods, biophysicists to develop and provide adapted experimental and theoretical models, chemist to design adapted probes and cell biologists. In collaboration with other teams of U1143 and the help of dedicated engineers (to be recruited) who will stimulate the interface between experiment and data sciences, we expect to build a general approach based on theories and tools in optics, chemistry, cell biology, biophysics, statistics, and machine learning.
Our case studies in cellular and chemical biology will be related to the analysis of intracellular transport and signaling pathways, and the migration of tumor cells in organoids, as they represent a major contributory factor to a number of diseases such as cancer and viral infection. For instance, we wish to study in detail the causal link between lectin-driven glycolipid reorganization in biological membranes and the formation of endocytic sites from which clathrin-independent endocytic carriers are generated. Since a series of pathogens (e.g., polyoma and noroviruses), pathogenic factors (e.g., Shiga and cholera toxins) and cellular proteins (integrins, CD44...) are concerned by this mechanism we expect that this study will have a general impact in the life science and membrane biophysics communities. Understanding and exploring diverse and alternative cellular entry mechanisms, by gathering as many as possible molecular information in fundamental membrane biology research, paves the way for the development of innovative cancer therapy or vaccine strategies. We expect that our results will be helpful in the design of therapeutic compounds delivered to precise intracellular locations within specialized cells for immunotherapy, or to tumors for targeted therapy.
Meanwhile, the ambition of SAIRPICO is to become the reference team in computational polarized bioimaging, with a focus on the development of advanced signal-image processing techniques for cell imaging. To that end, we will create a centralized polarized image database and disseminate the results through dedicated workshops, summer schools, mini-symposia, on-line tutorials, and publications in high-visibility journals. It is worth noting that the interdisciplinary team will be be-localized in Rennes and Paris and therefore will benefit from the scientific environment of both Inria (Applied mathematics, artificial intelligence) and Institut Curie (chemical biology, optics).
5 Social and environmental responsibility
Cancer is the second most common cause of death in EU countries, after cardiovascular disease, and Europe accounts for a quarter of all cancer cases worldwide, despite representing less than 10% of the world's population: it is therefore clear that cancer has a considerable impact on our society, putting pressure on national healthcare and social protection systems, public budgets and economic growth. Research policies in cancer control and diagnosis are increasingly based on results obtained in artificial intelligence applied to cellular imaging. Action to prevent cancer also contributes to the fight against obesity and other diseases such as cardiovascular disease and diabetes, since they share common risk factors.
6 Highlights of the year
6.1 Awards
Charles Kervrann received the 2024 Outstanding Editorial Board Member Award (service for the "IEEE Transactions on Image Processing" journal).
6.2 Publications
-
–
S. Herbreteau, C. Kervrann. Linear combinations of patches are unreasonably effective for single image denoising, IEEE Trans. Image Processing, 33: 4600-4613, 2024 – In this paper, we show that linear combinations of patches are enough to achieve state-of-the-art performance in image denoising. Although conceptually very simple, our method may be considered as the best single-image denoiser, outperforming the recent neural network-based techniques, while being much faster and fully interpretable.
-
–
MacDonald E, Forrester A, Valades Cruz CA, Madsen TD, Hetmanski JHR, Dransart E, Yeap N, Godbole R, Akhil Shp A, Leconte L, Chambon V, Ghosh D, Pinet A, Bhatia D, Lombard B, Loew D, Larsen MR, Leffler H, Lefeber DJ, Clausen H, Blangy A, Caswell P, Shafaq-Zadah M, Mayor S, Weigert R, Wunder C, and Johannes L. Growth factor-triggered desialylation controls glycolipid-lectin driven endocytosis. Accepted in Nature Cell Biology – In this paper, we demonstrate that growth factors (focus on EGF) trigger the acute remodeling (desialylation) of glycans on cell surface glycoproteins leading to their GL-Lect driven endocytosis, retrograde trafficking to the Golgi, resialylation, and polarized secretion to the leading edge to sustain cell migration. Our findings challenge the current dogma that glycans are mature and static once they have reached the plasma membrane. They open numerous perspectives on tuning protein function in biological processes.
7 New software, platforms, open data
7.1 New software
7.1.1 DCT2Net
-
Name:
Trained shallow CNN (convolution neural network)-based DCT (Discrete Cosine Transform) denoiser
-
Keywords:
Deep learning, Denoising, Convolutional Neural Network, Deconvolution
-
Functional Description:
DCT2net software, based on the well-known DCT (Discrete Cosine Transform) image denoising algorithm, is dedicated to noise removal from images. The traditional DCT denoiser can be seen as a shallow CNN and thereby its original linear transform can be tuned through gradient descent in a supervised manner, improving considerably its performance. Consequently, DCT2net is a shallow and interpretable convolution network, whose parameters optimization allows to improve very significantly the performances of the traditional DCT denoiser. To deal with the remaining artifacts induced by DCT2net, an original hybrid solution between DCT and DCT2net is proposed, combining the best of what these two methods can offer. Experiments on artificially noisy images show that the two-layer DCT2net method provides results comparable to the BM3D method and is as fast as the DnCNN algorithm composed of more than a dozen of layers.
Inter Deposit Digital Number: IDDN.FR.001.460033.000.S.P.2021.000.21000 21
- URL:
- Publication:
-
Contact:
Charles Kervrann
-
Participants:
Sebastien Herbreteau, Charles Kervrann, Leo Maury
7.1.2 DeepFinder
-
Name:
Deep learning for macromolecule identification within 3D cellular cryo-electron tomograms
-
Keywords:
Image analysis, Deep learning, Cryo-electron microscopy, Object detection
-
Functional Description:
DeepFinder is a computational approach that uses artificial neural networks to accurately and jointly localize multiple types and/or states of macromolecules in 3D cellular cryo-electron tomograms. DeepFinder leverages deep learning and outperforms the commonly-used template matching method on ideal data. On synthetic image data (SHREC 2019, 2020, and 2021 challenges), DeepFinder is very fast and produces superior detection results when compared to other competitive deep learning methods, especially on small macromolecules. On experimental cryo-ET data depicting ribosomes, the detection results obtained by DeepFinder are consistent with expert annotations. We have got a high overlap of detection (86%) and a similar structure resolution that those determined by subtomogram averaging.
Inter Deposit Digital Number: IDDN.FR.001.460030.000.S.P.2021.000.21000
- URL:
- Publication:
-
Contact:
Emmanuel Moebel
-
Participants:
Arthur Masson, Mounir Messaoudi, Emmanuel Moebel, Charles Kervrann
-
Partners:
Max Planck Institute Martinsried, Fondation Fourmentin-Guilbert, Helmholtz Pioneer Campus, Université de Strasbourg
7.1.3 GcoPS
-
Name:
Geo-Co-Positioning System for co-localization of image pairs of fluorescent molecules
-
Keywords:
Photonic imaging, Fluorescence microscopy, Image processing, Statistic analysis
-
Functional Description:
The GcoPS (Geo-Co-Positioning System) software is dedicated to the co-localization of fluorescence image pairs for both conventional and super-resolution microscopy. The procedure is only controlled by a p-value (type I error-rate) and tests whether the Pearson correlation between two binary images is significantly positive. Colocalization amounts here to quantifying the interaction strength by the area/volume of the intersection between the two binary images viewed as random distributions of geometrical objects. Under mild assumptions, it turns out that the appropriately normalized Pearson correlation follows a standard normal distribution under the null hypothesis if the number of image pixels is large. Unlike previous methods, GcoPS handles 2D and 3D images, variable SNRs and any kind of cell shapes. It is able to co-localize large regions with small dots, as it is the case in TIRF-PALM experiments and to detect negative co-localization. The typical processing time is two milliseconds per image pair in 2D and a few seconds in 3D, with no dependence on the number of objects per image. In addition, the method provides maps to geo-co-localize molecule interactions in specific image regions.
- Publication:
-
Contact:
Charles Kervrann
-
Participants:
Charles Kervrann, Arthur Masson, Thierry Pécot, Frédéric Lavancier, Florent Leray
-
Partners:
Université de Nantes, UMR 144 CNRS - Institut Curie, Institut de Génétique & Développement de Rennes, ENSAI
7.1.4 ExoDeepFinder
-
Name:
A Deep learning method for exocytosis event detection in fluorescence TIRF microscopy movies
-
Keywords:
Image analysis, Deep learning, Fluorescence microscopy, Live-cell microscopy, Anomaly detection
-
Functional Description:
ExoDeepFinder is a software for the detection of rare dynamic exocytosis events observed in temporal series of 2D Total Internal Reflection Fluorescent Microscopy (TIRFM) images. This U-net, originally designed for analyzing 3D cryo-electron tomography images (DeepFinder), achieved good absolute performances with a relatively small training dataset of 60 cells/2000 events. ExoDeepFinder method uses hybrid annotations performed manually by experts (more than 10, 000 spatiotemporal coordinates of annotated exocytosis events) and automatically annotated bright spots that are not bona fide exocytosis events, with no data curation. By gathering the manual and automatic datasets, we significantly boosted the performance in order to detect very rare events in the volumes (< 1 event per frame in average), even if the automatic spot detector (ATLAS) potential produces annotation errors. ExoDeepFinder outcompeted the unsupervised conventional methods on a benchmark composed of several dozen experimental movies of one thousand frames with variable signal-to-background ratios, while exhibiting a greater plasticity to the experimental conditions when tested under drug treatments and after changes in cell line or imaged reporter. This robustness to unseen experimental conditions did not require re-training demonstrating generalization capability of ExoDeepFinder. The algorithm, designed for large 2D+time volume processing, takes about 30 seconds to process a video of 300 x 300 x 1000 voxels with no parameter adjustment, as compared to 10 to 20 minutes required with the two conventional image analysis algorithms. The training needs 8 to 16 hours at most of computing (once for all), depending on the desired number of epochs and GPU performance. The method, as well as the annotated training datasets, were made transparent and available through an open-source software as well as a Napari plugin and can directly be applied to custom user data. The apparent plasticity and performances of ExoDeepFinder to detect dynamic events open new opportunities for future deep-learning guided analysis of dynamic processes in live-cell imaging such as membrane trafficking, synaptic biology or secretion.
- URL:
- Publication:
-
Contact:
Arthur Masson
-
Participants:
Charles Kervrann, Arthur Masson, Hugo Lachuer, Anne-Sophie Mace, Kristin Schauer, Emmanuel Moebel
-
Partners:
Institut Jacques Monod, Institut Gustave Roussy, UMR 144 CNRS - Institut Curie
7.1.5 LIChI
-
Name:
Linear and Iterative Combinations of patches for Image denoising
-
Keywords:
Image analysis, Denoising
-
Functional Description:
In the past decade, deep neural networks have revolutionized image denoising in achieving significant accuracy improvements by learning on datasets composed of noisy/clean image pairs. However, this strategy is extremely dependent on training data quality, which is a well-established weakness. To alleviate the requirement to learn image priors externally, single image (a.k.a., self-supervised or zero-shot) methods perform denoising solely based the analysis of the input noisy image without external dictionary or training dataset. This work investigates the effectiveness of linear combinations of patches for denoising under this constraint. Although conceptually very simple, we show that linear combinations of patches are enough to achieve state-of-the-art performance. The proposed parametric approach relies on quadratic risk approximation via multiple pilot images to guide the estimation of the combination weights. Experiments on images corrupted artificially with Gaussian noise as well as on real-world noisy images demonstrate that our method is on par with the very best single-image denoisers, outperforming the recent neural network-based techniques, while being much faster and fully interpretable.
- URL:
- Publication:
-
Contact:
Charles Kervrann
-
Participants:
Sebastien Herbreteau, Charles Kervrann
-
Partner:
Airbus Defense and Space
7.1.6 NL-Ridge
-
Name:
A unified framework of non-local parametricmethods for image denoising
-
Keywords:
Image analysis, Denoising
-
Functional Description:
We propose a unified view of non-localmethods for single-image denoising, for which BM3D is the most popular representative, that operate by gathering noisy patches together according to their similarities in order to process them collaboratively. Our general estimation framework is based on the minimization of the quadratic risk, which is approximated in two steps, and adapts to photon and electronic noises. Relying on unbiased risk estimation (URE) for the first step and on “internal adaptation”, a concept borrowed from deep learning theory, for the second, we show that our approach enables to reinterpret and reconcile previous state-of-the-art non-local methods. Within this framework, we propose a novel denoiser called NL-Ridge that exploits linear combinations of patches. While conceptually simpler, we show that NL-Ridge can outperform well-established state-of-the-art single-image denoisers.
- URL:
- Publication:
-
Contact:
Charles Kervrann
-
Participants:
Sebastien Herbreteau, Charles Kervrann
-
Partner:
Airbus Defense and Space
7.1.7 DeepCristae
-
Name:
A CNN for the restoration of mitochondria cristae in live microscopy images
-
Keywords:
Image analysis, Deep learning, Deconvolution, Denoising, Live-cell microscopy, Fluorescence microscopy, Convolutional Neural Network
-
Functional Description:
DeepCristae is a CNN specifically developed to restore mitochondria cristae in low spatial resolution microscopy images. The main specificities of the method are 1) a new training loss dedicated to the restoration of specific pixels of interest, 2) a random image patch sampling focusing on areas of mitochondria to increase the size of the training set, and 3) metrics for objective assessment of cristae restoration. DeepCristae was applied to several microscopy modalities and different biological scenarios capturing live mitochondria at high speed with low illumination and thus low phototoxicity. It allows long-term/fast dynamic observation of cristae behavior and organization.
- URL:
- Publication:
-
Contact:
Anais Badoual
-
Participants:
Anais Badoual, Ludovic Leconte, Cesar Augusto Valades Cruz, Jean Salamero, Salome Papereux, Charles Kervrann
-
Partner:
UMR 144 CNRS - Institut Curie
7.1.8 BDM-Generator4BioImaging
-
Name:
A generative "Birth-Death-Move" model to simulate spatiotemporal dynamics of biomolecules in cells
-
Keywords:
Live-cell microscopy, Stochastic models, Image analysis, Multi-Object Tracking, Multi-physics simulation, Marked Point Process
-
Functional Description:
Generators of space-time dynamics in bioimaging have become essential to build ground truth datasets for image processing algorithm evaluation such as biomolecule detectors and trackers, as well as to generate training datasets for deep learning algorithms. In this contribution, we leverage a stochastic model, called birth-death-move (BDM) point process, in order to generate joint dynamics of biomolecules in cells. This particle-based stochastic simulation method is very flexible and can be seen as a generalization of well-established standard particle-based generators. In comparison, our approach allows us: (1) to model a system of particles in motion, possibly in interaction, that can each possibly switch from a motion regime (e.g., Brownian) to another (e.g., a directed motion), (2) to take into account finely the appearance over time of new trajectories and their disappearance, these events possibly depending on the cell regions but also on the current spatial configuration of all existing particles. This flexibility enables to generate more realistic dynamics than standard particle-based simulation procedures, by for example accounting for the colocalization phenomena often observed between intracellular vesicles.
- URL:
- Publication:
-
Contact:
Frédéric Lavancier
-
Participants:
Lisa Balsollier, Frédéric Lavancier, Charles Kervrann
-
Partners:
Université de Nantes, ENSAI, UMR 144 CNRS - Institut Curie
7.1.9 ST-Space-Time-Flow-segmentation
-
Name:
Unsupervised space-time network for temporally-consistent segmentation of multiple motions
-
Keywords:
Neural networks, Unsupervised learning, Optic-flow, Image sequence, Motion estimation, Segmentation
-
Functional Description:
Unsupervised segmentation of the optical flow by convolutional neural networks, taking into account the temporal dimension. The software takes an optic flow volume as input and provides an optic flow segmentation volume as output. Inference is performed in a single iteration. The software includes the trained weights of the network.
- URL:
- Publication:
-
Contact:
Etienne Meunier
-
Participants:
Etienne Meunier, Patrick Bouthemy
7.2 New platforms
Participants: Charles Kervrann, Arthur Masson.
BioImageIT for bioimage management and processing –
New image acquisition systems generate large number of images and large volume images. Such data sets are hard to store, to process and to analyze for in a workstation. Many solutions exist for data management (e.g. Omero, OpenImadis), image analysis (e.g. Fiji, Icy, CellProfiler) and statistics (e.g R software). Each of them has its specificities and several bridges have been developed between pieces of software. Nevertheless, in many use-cases, we need to perform analysis using tools that are available in different pieces of software and different languages. It is then tedious to create a workflow that brings the data from one tool to another. This process requires programming skills and most of the time, custom scripts are developed to handle data processing management. To overcome these difficulties, we have already developed a framework – BioImageIT (bioimageit.github.io) – to create a middleware application that allow any scientist to annotate, process, and analyze data using only one single high level application. This BioImageIT application is based on 3 components:
- an image annotation method interoperable with existing databases;
- an image processing and analysis tools integration method based on packaging and wrapping techniques;
- an application with a graphical interface to easily annotate data, run processing tools, and visualize data and results.
This software architecture has three main goals. First, data are annotated with open formats and experiment can then be stored in different architectures or servers. Second, the processing tools are used as binary packages managed by the Conda or Docker technologies. These technologies enable to gently handle dependencies and several versions of the same tool. Any existing tool can then be integrated in its native programming language. Third, using a single middleware application allows to automatically generate metadata for any processed data, improving the traceability and the repeatability of any experimental result (FAIR principles).
We envision to continue to promote BioImageIT in the forthcoming years, initiated in the frame of the France-BioImaging research infrastructure (france-bioimaging.org) in order to provide a standardized image processing tool set and data management for the imaging facilities.
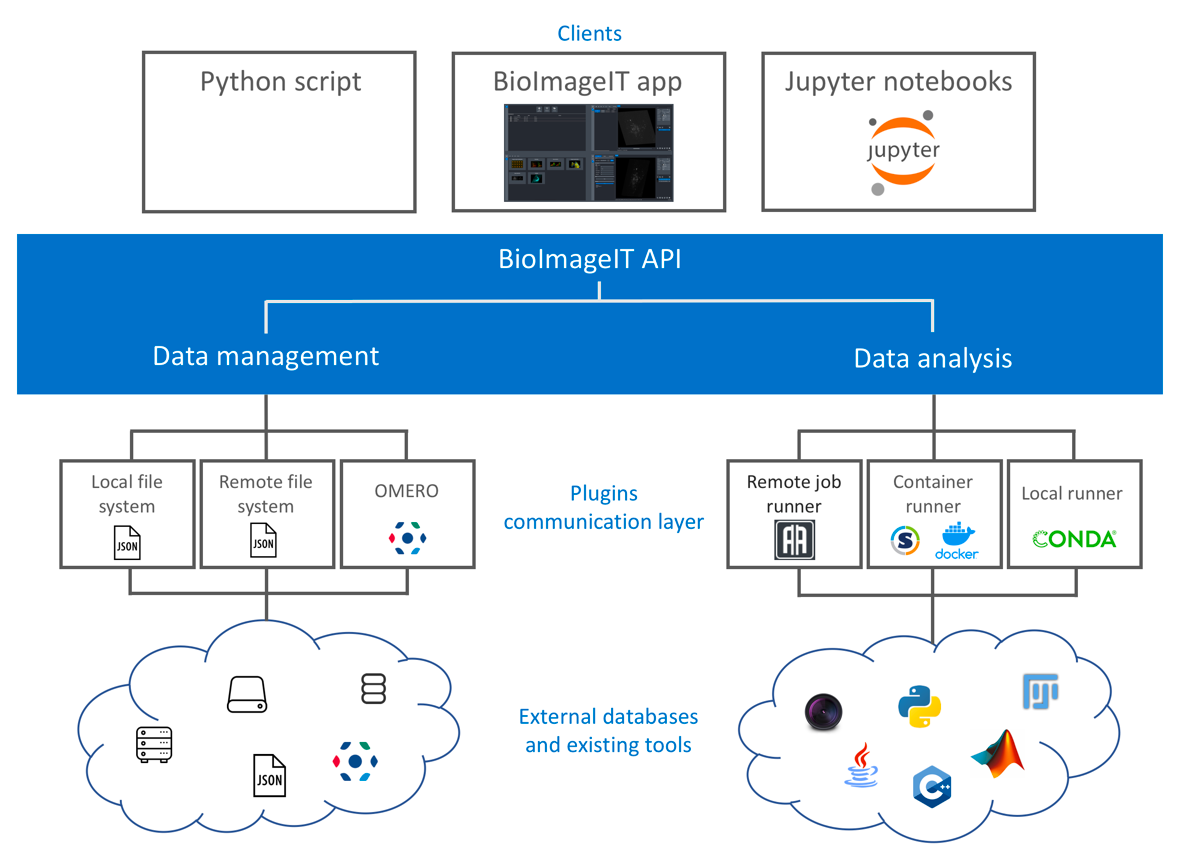
Scheme of the BioImageIT components interactions
8 New results
Note: In this section, we provide details of the "scientific production". Each paragraph summarizes a published or submitted paper.
8.1 Supervised/unsupervised methods for image restoration and object detection in imaging and microscopy
DeepCristae, a CNN for the restoration of mitochondria cristae in live microscopy images
Participants: Anaïs Badoual, Charles Kervrann.
Mitochondria play an essential role in the life cycle of eukaryotic cells. However, we still don't know how their ultrastructure, like the cristae of the inner membrane, dynamically evolves to regulate these fundamental functions, in response to external conditions or during interaction with other cell components. Although high-resolution fluorescent microscopy coupled with recently developed innovative probes can reveal this structural organization, their long-term, fast and live 3D imaging remains challenging. To address this problem, we have developed a convolutional neural network (CNN), called DeepCristae, to restore mitochondrial cristae in low spatial resolution microscopy images. Our CNN is trained from 2D STED images using a novel loss specifically designed for cristae restoration. Random sampling centered on mitochondrial areas was also developed to improve training efficiency. Quantitative assessments were carried out using metrics we derived to give a meaningful measure of cristae restoration. Depending on the conditions of use indicated, DeepCristae works well on broad microscopy modalities (STED, Live-SR, AiryScan and LLSM). It is ultimately applied in the context of mitochondrial network dynamics during interaction with endo/lysosomes membranes. (in collaboration with J. Salamero, L. Leconte, C.A. Valades-Cruz, CNRS-UMR144, Institut Curie; T. Liu, Z. Chen, PKU University, Institute of Molecular Medicine, Beijing, People Republic of China)
S. Papereux, L. Leconte, C.A. Valades-Cruz, T. Liu, J. Dumont, Z. Chen, J. Salamero, C. Kervrann, A. Badoual. DeepCristae, a CNN for the restoration of mitochondria cristae in live microscopy images, 2024, bioRxiv-DOI:10.1101/2023.07.05.547594, hal-04295317. (to appear in "Communications Biology")
Linear combinations of patches are unreasonably effective for single image denoising
Participant: Charles Kervrann.
In the past decade, deep neural networks have revolutionized image denoising in achieving significant accuracy improvements by learning on datasets composed of noisy/clean image pairs. However, this strategy is extremely dependent on training data quality, which is a well-established weakness. To alleviate the requirement to learn image priors externally, single-image (a.k.a., self-supervised or zero-shot) methods perform denoising solely based on the analysis of the input noisy image without external dictionary or training dataset. This work investigates the effectiveness of linear combinations of patches for denoising under this constraint. Although conceptually very simple, we show that linear combinations of patches are enough to achieve state-of-the-art performance. The proposed parametric approach relies on quadratic risk approximation via multiple pilot images to guide the estimation of the combination weights. Experiments on images corrupted artificially with Gaussian noise as well as on real-world noisy images demonstrate that our method is on par with the very best single-image denoisers, outperforming the recent neural network-based techniques, while being much faster and fully interpretable. (in collaboration with S. Herbreteau, ENSAI, Bruz, France; R. Fraisse, AIRBUS Defence and Space, Toulouse, France)
S. Herbreteau, C. Kervrann. Linear combinations of patches are unreasonably effective for single image denoising, IEEE Trans. Image Processing, 33: 4600-4613, 2024, DOI:10.1109/TIP.2024.3436651, hal-03894346. (LIChI software)
A unified framework of non-local parametric methods for image denoising
Participant: Charles Kervrann.
We propose a unified view of non-local methods for single-image denoising, for which BM3D is the most popular representative, that operate by gathering noisy patches together according to their similarities in order to process them collaboratively. Our general estimation framework is based on the minimization of the quadratic risk, which is approximated in two steps, and adapts to photon and electronic noises. Relying on unbiased risk estimation (URE) for the first step and on “internal adaptation”, a concept borrowed from deep learning theory, for the second, we show that our approach enables to reinterpret and reconcile previous state-of-the-art non-local methods. Within this framework, we propose a novel denoiser called NL-Ridge that exploits linear combinations of patches. While conceptually simpler, we show that NL-Ridge can outperform well-established state-of-the-art single-image denoisers. (in collaboration with S. Herbreteau, ENSAI, Bruz, France; R. Fraisse, AIRBUS Defence and Space, Toulouse, France)
S. Herbreteau, C. Kervrann. A unified framework of non-local parametric methods for image denoising. SIAM J. Imaging Sciences, 18(1): 89-119, 2025, DOI:10.1137/24M1630967, hal-04472406. (NL-Ridge software)
On normalization-equivariance properties of supervised and unsupervised denoising methods: a survey
Participant: Charles Kervrann.
Image denoising is probably the oldest and still one of the most active research topic in image processing. Many methodological concepts have been introduced in the past decades and have improved performances significantly in recent years, especially with the emergence of convolutional neural networks and supervised deep learning. In this paper, we propose a survey of guided tour of supervised and unsupervised learning methods for image denoising, classifying the main principles elaborated during this evolution, with a particular concern given to recent developments in supervised learning. It is conceived as a tutorial organizing in a comprehensive framework current approaches. We give insights on the rationales and limitations of the most performant methods in the literature, and we highlight the common features between many of them. Finally, we focus on on the normalization equivariance properties that is surprisingly not guaranteed with most of supervised methods. It is of paramount importance that intensity shifting or scaling applied to the input image results in a corresponding change in the denoiser output. (in collaboration with S. Herbreteau, ENSAI, Bruz, France; R. Fraisse, AIRBUS Defence and Space, Toulouse, France)
S. Herbreteau, C. Kervrann. On normalization-equivariance properties of supervised and unsupervised denoising methods: a survey, 2024, hal-04477109. (submitted to "Int. J. Computer Vision")
8.2 Supervised deep-learning for detection, segmentation, classification, and motion analysis in imaging
Template Learning: deep learning with domain randomization for particle picking in cryo-electron tomography
Participant: Charles Kervrann.
Cryo-electron tomography (cryo-ET) enables the three-dimensional visualization of biomolecules and cellular components in their near-native state. Particle picking, a crucial step in cryo-ET data analysis, is traditionally performed by template matching—a method utilizing cross-correlations with available biomolecular templates. Despite the effectiveness of recent deep learning-based particle picking approaches, their dependence on initial data annotation datasets for supervised training remains a significant limitation. Here, we propose a technique that combines the accuracy of deep learning particle identification with the convenience of the model training on biomolecular templates enabled through a tailored domain randomization approach. Our technique, named Template Learning, automates the simulation of training datasets, incorporating considerations for molecular crowding, structural variabilities, and data acquisition variations. This reduces or even eliminates the dependence of supervised deep learning on annotated experimental datasets. We demonstrate that models trained on simulated datasets, optionally fine-tuned on experimental datasets, outperform those exclusively trained on experimental datasets. Also, we illustrate that Template Learning used as an alternative to template matching, can offer higher precision and better orientational isotropy, especially for picking small non-spherical particles. Template Learning software is open-source, Python-based, and GPU and CPU parallelized. (in collaboration with M. Eltsov and M. Harastani, IGBMC Strasbourg)
M. Harastani, G. Patra, C. Kervrann, M. Eltsov. Template Learning: deep learning with domain randomization for particle picking in cryo-electron tomography, 2024, BioRxiv-DOI:10.1101/2024.03.20.585905, hal-04874266. (submitted to "Nature Communications", in revision)
Deep learning detection of dynamic exocytosis events in fluorescence TIRF microscopy
Participants: Charles Kervrann, Arthur Masson.
Segmentation and detection of biological objects in fluorescence microscopy is of paramount importance in cell imaging. Deep learning approaches have recently shown promise to advance, automatize and accelerate analysis. However, most of the interest has been given to the segmentation of static objects of 2D/3D images whereas the segmentation of dynamic processes obtained from time-lapse acquisitions has been less explored. Here we adapted DeepFinder, a U-net originally designed for 3D noisy cryo-electron tomography (cryo-ET) data, for the detection of rare dynamic exocytosis events (termed ExoDeepFinder) observed in temporal series of 2D Total Internal Reflection Fluorescent Microscopy (TIRFM) images. ExoDeepFinder achieved good absolute performances with a relatively small training dataset of 60 cells/12000 events. We rigorously compared deep learning performances with unsupervised conventional methods from the literature. ExoDeepFinder outcompeted the tested methods, but also exhibited a greater plasticity to the experimental conditions when tested under drug treatments and after changes in cell line or imaged reporter. This robustness to unseen experimental conditions did not require re-training demonstrating generalization capability of ExoDeepFinder. ExoDeepFinder, as well as the annotated training datasets, were made transparent and available through an open-source software as well as a Napari plugin and can directly be applied to custom user data. The apparent plasticity and performances of ExoDeepFinder to detect dynamic events open new opportunities for future deep-learning guided analysis of dynamic processes in live-cell imaging.(in collaboration with H. Lachuer, Institut Jacques Monod; K. Schauer, Institut Gustave-Roussy; A.S. Macé, Institut Curie)
H. Lachuer, E. Moebel, A.S. Macé, A. Masson, K. Schauer, C. Kervrann. Deep learning detection of dynamic exocytosis events in fluorescence TIRF microscopy, 2024, BioRxiv-DOI:10.1101/2024.09.09.611975, hal-04874728. (submitted to "PLoS Computational Biology")
Ensembling Unets for rare chromosomal aberration detection in metaphase images, uncertainty quantification, and ionizing radiation dose estimation
Participant: Charles Kervrann.
In biological dosimetry a radiation dose is estimated using the average number of chromosomal aberrations per peripheral blood lymphocytes. This analysis is still manually performed on 2D metaphase images depicting the 23 pairs of chromosomes because the false discovery rate of current automated detection systems is too high and variable because of sensitivity to small variations in image quality (chromosome spread, illumination variations ...). Therefore, the current systems are only used to assist human experts. Designing more performant automatic and reliable chromosomal aberration detection systems has become of paramount importance to improve diagnosis speed and reduce human expertise time. Here, we propose a novel deep-learning method for automatic rare chromosomal aberration detection and uncertainty quantification. We formulate the problem as a unique regression problem requiring the minimization of a sparsity-promoting loss to reduce the false alarm rate. Furthermore, we select checkpoints at the end of each epoch during training to form a model ensemble. The resulting artificial experts are further analyzed to derive a consensus voting, similar to an agreement of human annotator rating, to provide trustworthy aberration detections and confidence intervals. A radiation dose curve is finally derived from deep learning-assisted counting of dicentrics and fragments in metaphase images, in high agreement with the reference hand-crafted curve in biological dosimetry. (in collaboration with M.A. Benadjaoud, IRSN/PSE-SANTE/SERAMED/LRAcc)
A. Deschemps, E. Grégoire, J.S. Martinez, A. Vaurijoux, P. Fernandez, D. Dugue, L. Bobyk, M. Valente, G. Gruel, E. Moebel, M.A. Benadjaoud, C. Kervrann. Ensembling Unets for rare chromosomal aberration detection in metaphase images, uncertainty quantification, and ionizing radiation dose estimation, 2024, hal-04874432. (submitted to "Biomedical Signal Processsing and Control")
Diffusion model uniform manifold filtering for classification of small datasets with underrepresented classes: Application to chromosomal aberration microscopy detection
Participants: Quentin Tallon, Charles Kervrann.
In biological dosimetry a radiation dose is estimated using the average number of chromosomal aberrations per peripheral blood lymphocytes. This analysis is still manually performed on 2D metaphase images depicting the 23 pairs of chromosomes because the false discovery rate of current automated detection systems is too high and variable because of sensitivity to small variations in image quality (chromosome spread, illumination variations ...). Therefore, the current systems are only used to assist human experts. Designing more performant automatic and reliable chromosomal aberration detection systems has become of paramount importance to improve diagnosis speed and reduce human expertise time. Here, we propose a novel deep-learning method for automatic rare chromosomal aberration detection and uncertainty quantification. We formulate the problem as a unique regression problem requiring the minimization of a sparsity-promoting loss to reduce the false alarm rate. Furthermore, we select checkpoints at the end of each epoch during training to form a model ensemble. The resulting artificial experts are further analyzed to derive a consensus voting, similar to an agreement of human annotator rating, to provide trustworthy aberration detections and confidence intervals. A radiation dose curve is finally derived from deep learning-assisted counting of dicentrics and fragments in metaphase images, in high agreement with the reference hand-crafted curve in biological dosimetry. (in collaboration with M.A. Benadjaoud, IRSN/PSE-SANTE/SERAMED/LRAcc)
Q. Tallon, J.S. Martinez, E. Grégoire, P. Fernandez, D. Dugue, G. Gruel, C. Kervrann, M.A. Benadjaoud. Diffusion model uniform manifold filtering for classification of small datasets with underrepresented classes: Application to chromosomal aberration microscopy detection. In Proc. Int. Conf. Machine Vision (ICMV), Edimburg, Scottland, 2024, hal-04900898. (Best Presentation Award)
Early prediction of the transferability of bovine embryos from video-microscopy
Participant: Patrick Bouthemy.
Videomicroscopy is a promising tool combined with machine learning for studying the early development of in vitro fertilized bovine embryos and assessing its transferability as soon as possible. We aim to predict the embryo transferability within four days at most, taking 2D time-lapse microscopy videos as input. We formulate this problem as a supervised binary classification problem for the classes transferable and not transferable. The challenges are three-fold: 1) poorly discriminating appearance and motion, 2) class ambiguity, 3) small amount of annotated data. We propose a 3D convolutional neural network involving three pathways, which makes it multi-scale in time and able to handle appearance and motion in different ways. For training, we retain the focal loss. Our model, named SFR, compares favorably to other methods. Experiments demonstrate its effectiveness and accuracy for our challenging biological task. (in collaboration with E. Fromont and Y. Hachani, Lacodam Team, IRISA, Rennes; A. De Paula Reis, UMR BREED, Ecole Nationale Vétérinaire d’Alfort)
Y. Hachani, P. Bouthemy, E. Fromont, S. Ruffini, L. Laffont and A. De Paula Reis, Early prediction of the transferability of bovine embryos from videomicroscopy, In Proc. IEEE Int. Conf. Image Processing (ICIP), Abu Dhabi, United Arab Emirates, pp. 2149-2155, 2024, DOI:10.1109/ICIP51287.2024.10647901, hal-04880222.
Y. Hachani, P. Bouthemy, S. Ruffini, L. Laffont, E. Fromont and A. d. P. Reis. Prédiction précoce de la transférabilité d’embryons bovins par vidéomicroscopie, In Proc. Congrès Reconnaissance des Formes, Image, Apprentissage et Perception (RFIAP 2024), Lille, France, 2024, hal-04614044.
Prediction of active surfaces for 3D cell instances segmentation
Participants: Quentin Rapilly, Anaïs Badoual, Charles Kervrann.
Multi-instance segmentation algorithms are of great interest in a very large range of fields. Deep learning brought major improvements in terms of processing speed or prediction accuracy. Nevertheless, some traditional methods such as active surfaces have features that conventional deep learning methods cannot provide, especially representing the object in a continuous geometrical way and encoding prior information on the shapes to segment. Those features are of particular interest in biology to efficiently segment noisy and poorly resolved data, and then understand the interactions between segmented cells. Here, we introduce a new hybrid segmentation method dedicated to multi-instance segmentation of 3D images that combines the efficiency of deep learning and the powerful representation of active surfaces. We evaluate our method on real and synthetic 3D datasets of fluorescence microscopy.
Q. Rapilly, A. Badoual and C. Kervrann. Prediction of active surfaces for 3D cell instances segmentation, In Proc. Congrès Reconnaissance des Formes, Image, Apprentissage et Perception (RFIAP 2024), Lille, France, 2024, hal-04611350.
Efficient local correlation volume for unsupervised optical flow estimation on small moving objects in large images
Participant: Patrick Bouthemy.
With the advent of deep learning methods performance and efficiency of optical flow estimation has significantly increased especially for supervised models. However they do not generalize well to more specific data involving small moving objects in large images such as high-resolution biologial or satellite sequences. In addition annotation and realistic simulation are difficult for these contents which calls for unsupervised alternatives. Yet the latter are still less accurate than their supervised counterparts. In this paper we introduce an unsupervised local optical flow estimation method adapted to small moving objects in large-size images by involving no downsampling of the feature maps. We adopt a local correlation search and implement it in an original way with a per-shift computation which minimizes memory consumption and speeds up inference computation for large-scale images. We also design a loss function combining similarity smoothness and sparsity constraints. We demonstrate the performance of our SMOFlow method on real stabilized aerial videos fully representative of future satellite conditions. SMOFlow favorably compares to other methods. Our SMOFlow method is able to accurately capture the motion of small objects in large images while efficiently reducing memory consumption. (in collaboration with R. Fraisse, AIRBUS Defence and Space)
S. Khairi, E. Meunier, R. Fraisse, P. Bouthemy. Efficient local correlation volume for unsupervised optical flow estimation on small moving objects in large images, in Proc. IEEE/CVF Computer Vision Pattern Recognition workshops (CVPRW), pp. 440-448, Seattle, WA, USA, 2024, DOI:10.1109/CVPRW63382.2024.00049, hal-04907647.
Parametric estimation and LAN property of the birth-death-move process with mutations
Participants: Lisa Balsollier, Frédéric Lavancier.
A birth-death-move process with mutations is a Markov model for a system of marked particles in interaction, that move over time, with births and deaths. In addition the mark of each particle may also change, which constitutes a mutation. Assuming a parametric form for this model, we derive its likelihood expression and prove its local asymptotic normality. The efficiency and asymptotic distribution of the maximum likelihood estimator, with an explicit expression of its covariance matrix, is deduced. The underlying technical assumptions are showed to be satisfied by several natural parametric specifications. As an application, we leverage this model to analyse the joint dynamics of two types of proteins in a living cell, that are involved in the exocytosis process. Our approach enables to quantify the so-called colocalization phenomenon, answering an important question in microbiology. (in collaboration with F. Lavancier, ENSAI, Bruz)
L. Balsollier, F. Lavancier. Parametric estimation and LAN property of the birth-death-move process with mutations, 2024, ArXiv-DOI:10.48550/arXiv.2404.19367, hal-04554652.
8.3 Analysis of spatiotemporal biological mechanisms and processes
Endocytic roles of glycans on proteins and lipids
Participants: Massiullah Shafaq-Zadah, Estelle Dransart, Christian Wunder, Ludger Johannes.
Most cell surface proteins are decorated by glycans, and the plasma membrane is rich in glycosylated lipids. The mechanisms by which the enormous complexity of these glycan structures on proteins and lipids is exploited to control glycoprotein activity by setting their cell surface residence time and the ways by which they are taken up into cells are still under active investigation. Here, two mechanisms are presented, termed galectin lattices and glycolipid-lectin (GL-Lect)-driven endocytosis, which are among the most prominent to establish a link between glycan information and endocytosis. Types of glycans on glycoproteins and glycolipids are reviewed from the angle of their interaction with glycan-binding proteins that are at the heart of galectin lattices and GL-Lect-driven endocytosis. Examples are given to show how these mechanisms affect cellular functions ranging from cell migration and signaling to vascularization and immune modulation. Finally, outstanding challenges on the link between glycosylation and endocytosis are discussed.(in collaboration with H. Leffler, Lund University, Division of Microbiology, Immunology and Glycobiology, Sweden)
L. Johannes, M. Shafaq-Zadah, E. Dransart, C. Wunder, H. Leffler. Endocytic roles of glycans on proteins and lipids, Cold Spring Harb Perspect Biol., 16(1):a041398, 2024, DOI:10.1101/cshperspect.a041398.
Exploration into Galectin-3 driven endocytosis and lattices
Participants: Massiullah Shafaq-Zadah, Estelle Dransart, Ludger Johannes.
Essentially all plasma membrane proteins are glycosylated, and their activity is regulated by tuning their cell surface dynamics. This is achieved by glycan-binding proteins of the galectin family that either retain glycoproteins within lattices or drive their endocytic uptake via the clathrin-independent glycolipid-lectin (GL-Lect) mechanism. Here, we have used immunofluorescence-based assays to analyze how lattice and GL-Lect mechanisms affect the internalization of the cell adhesion and migration glycoprotein α5β1 integrin. In retinal pigment epithelial (RPE-1) cells, internalized α5β1 integrin is found in small peripheral endosomes under unperturbed conditions. Pharmacological compounds were used to competitively inhibit one of the galectin family members, galectin-3 (Gal3), or to inhibit the expression of glycosphingolipids, both of which are the fabric of the GL-Lect mechanism. We found that under acute inhibition conditions, endocytic uptake of α5β1 integrin was strongly reduced, in agreement with previous studies on the GL-Lect driven internalization of the protein. In contrast, upon prolonged inhibitor treatment, the uptake of α5β1 integrin was increased, and the protein was now internalized by alternative pathways into large perinuclear endosomes. Our findings suggest that under these prolonged inhibitor treatment conditions, α5β1 integrin containing galectin lattices are dissociated, leading to an altered endocytic compartmentalization. (in collaboration with H. Leffler, Lund University, Division of Microbiology, Immunology and Glycobiology, Sweden)
M. Shafaq-Zadah, E. Dransart, S.K. Mani, J.L. Sampaio, L. Bouidghaghen, U. Nilsson, H. Leffler, L. Johannes. Exploration into Galectin-3 Driven Endocytosis and Lattices, Biomolecules, 14(9): 1169, 2024, DOI:10.3390/biom14091169.
Growth factor-triggered desialylation controls glycolipid-lectin driven endocytosis
Participants: Massiullah Shafaq-Zadah, Estelle Dransart, Christian Wunder, Ludger Johannes.
Glycolipid-lectin (GL-Lect) driven endocytosis controls the formation of clathrin-independent carriers (CLICs) and the internalization of various cargos such as integrin. Whether this process is regulated in a dynamic manner remained unexplored. Here, we demonstrate that within minutes, the epidermal growth factor triggers the galectin-driven endocytosis of cell surface glycoproteins, such as integrins, that are key regulators of cell adhesion and migration. The onset of this process, mediated by the Na+/H antiporter NHE-1 and the neuraminidases Neu1/3, requires the pH-triggered enzymatic removal of sialic acids whose presence otherwise prevents galectin binding. Desialylated glycoproteins are then retrogradely transported to the Golgi apparatus where their glycan makeup is reset to regulate EGF-dependent invasive cell migration. Further evidence is provided for a role of neuraminidases and galectin-3 in acidification-dependent bone resorption. Glycosylation at the cell surface thereby emerges as a dynamic and reversible regulatory post-translational modification that controls a highly adaptable trafficking pathway. (in collaboration with R. Weigert, CI-NIH Bethesda, USA; H. Clausen, University of Copenhagen, Department of Cellular and Molecular Medicine, Denmark; S. Mayor, National Centre for Biological Sciences, Bangalore, India; H. Leffler, Lund University, Division of Microbiology, Immunology and Glycobiology, Sweden)
E. MacDonald, A. Forrester, C.A. Valades-Cruz, T.D. Madsen, J. Hetmanski, E. Dransart, Y. Ng, R. Godbole, A. Akhil Shp, L. Leconte, V. Chambon, D. Ghosh, A. Pinet, D.D. Bhatia, B. Lombard, D. Loew, M.R. Larson, H. Leffler, D.J. Lefeber, H. Clausen, P. Caswell, M. Shafaq-Zadah, S. Mayor, R. Weigert, C. Wunder, L. Johannes. Growth factor-induced desialylation for the fast control of endocytosis, 2024, bioRxiv-DOI:10.1101/2023.09.12.557183v1. (to appear in "Nature Cell Biology")
Mammalian cell-based production of glycans, glycopeptides and glycomodules
Participants: Christian Wunder, Ludger Johannes.
Access to defined glycans and glycoconjugates is pivotal for discovery, dissection, and harnessing of a range of biological functions orchestrated by cellular glycosylation processes and the glycome. We previously employed genetic glycoengineering by nuclease-based gene editing to develop sustainable production of designer glycoprotein therapeutics and cell-based glycan arrays that display glycans in their natural context at the cell surface. However, access to human glycans in formats and quantities that allow structural studies of molecular interactions and use of glycans in biomedical applications currently rely on chemical and chemoenzymatic syntheses associated with considerable labor, waste, and costs. Here, we develop a sustainable and scalable method for production of glycans in glycoengineered mammalian cells by employing secreted Glycocarriers with repeat glycosylation acceptor sequence motifs for different glycans. The Glycocarrier technology provides a flexible production platform for glycans in different formats, including oligosaccharides, glycopeptides, and multimeric glycomodules, and offers wide opportunities for use in bioassays and biomedical applications. (in collaboration with H. Clausen, University of Copenhagen, Department of Cellular and Molecular Medicine, Denmark)
T. Jaroentomeechai, R. Karlsson, F. Goerdeler, F.K.Y. Teoh, M.N. Gronset, D. de Wit, Y.-H. Chen, S. Furukawa, V. Psomiadou, R. Hurtado-Guerrero, E.E. Vidal-Calvo, A. Salanti, T.J. Boltje, L.J. van den Bos, C. Wunder, L. Johannes, K.T. Schjoldager, H.J. Joshi, R.L. Miller, H. Clausen, S.Y. Vakhrushev, Y. Narimatsu. Mammalian cell-based production of glycans, glycopeptides and glycomodules, Nature Communications, vol. 15, No 1, pp. 9668, 2024, DOI:10.1038/s41467-024-53738-9.
Spatial N-glycan rearrangement on α5β1integrin nucleates galectin-3 oligomers to determine endocytic fate
Participants: Massiullah Shafaq-Zadah, Estelle Dransart, Christian Wunder, Ludger Johannes.
Membrane glycoproteins frequently adopt different conformations when altering between active and inactive states. Here, we discover a molecular switch that exploits dynamic spatial rearrangements of N-glycans during such conformational transitions to control protein function. For the conformationally switchable cell adhesion glycoprotein α 5 β 1 integrin, we find that only the bent-closed state arranges N-glycans to nucleate the formation of up to tetrameric oligomers of the glycan-binding protein galectin-3. We propose a structural model of how these galectin-3 oligomers are assembled and how they clamp the bent-closed state to prime it for endocytic uptake and subsequent retrograde trafficking to the Golgi for polarized distribution in cells. Our findings highlight an unexpectedly dynamic regulation of the glycan landscape at the cell surface to achieve oligomerization of galectin-3. Galectin-3 oligomers are thereby identified as decoders of defined spatial patterns of N-glycans and as functional extracellular interactors of specifically the bent-closed conformational state of α 5 β 1 integrin and possibly other family members. (in collaboration with H. Leffler, Lund University, Division of Microbiology, Immunology and Glycobiology, Sweden; D. Roderer and S. Raunser, Leibniz-Forschungsinstitut für Molekulare Pharmakologie, Berlin, Germany)
M. Shafaq-Zadah, E. Dransart, C. Wunder, V. Chambon, C.A. Valades-Cruz, L. Leconte, N.K. Sarangi, J. Robinson, S. Bai, R. Regmi, A.D. Cicco, A. Hovasse, R. Bartels, U.J. Nilsson, S. Cianférani-Sanglier, H. Leffler, T.E. Keyes, D. Lévy, S. Raunser, D. Roderer, L. Johannes. N-glycan rearrangement on α5β1integrin nucleates galectin-3 oligomers to determine endocytic fate, 2024, hal-04727374v1.
9 Bilateral contracts and grants with industry
9.1 Bilateral Grants with Industry
9.1.1 Contract with IRSN: DeepSuN – Localization of chromosomal aberrations induced by nuclear radiation dose excess
Participant: Charles Kervrann.
Funding: IRSN (Institut de Radioprotection et de Sureté Nucléaire) and Région-Bretagne
Duration: 48 months (Oct 2020 – Sep 2024)
Collaborator: M. Benadjaoud (IRSN/SERAMED, Fontenay-aux-Roses)
The goal of this project is to develop statistical and deep-learning methods for localizing and classifying chromosomal aberrations observed in 2D microscopy images (blood test) and estimating radiation dose following a postulated nuclear reactor accident.
This project funded by the IRSN (Institut de Radioprotection et de Sureté Nucléaire) and Région-Bretagne concerns the PhD thesis (co-funding) carried out by Antonin Deschemps. (see also ANR INCREASED)
9.1.2 Contract with AID: Localization and classification of gene translocation in FISH microscopy images
Participants: Quentin Tallon, Charles Kervrann.
Funding: AID (Agence de l'Innovation de Defense)
Duration: 36 months (Oct 2021 – Sep 2024)
Collaborator: M. Benadjaoud (IRSN/SERAMED, Fontenay-aux-Roses)
The goal of this project is to develop supervised and unsupervised machine learning methods and algorithms for detection and classification of gene translocation between chromosomes observed in FISH (Fluorescence in situ hybridization) microscopy images.
This project funded by the AID (Ministry of Defense) concerns the PhD thesis carried out by Quentin Tallon. (see also ANR INCREASED)
9.1.3 Contract with AIRBUS Defense and Space SAS: LION Chaine Image Elargie (LiChIE)
Participants: Patrick Bouthemy, Charles Kervrann.
Funding: Bpifrance / Projets Structurants pour la Compétitivité (PSPC)
Duration: 65 months (Aug 2019 – Dec 2024)
Collaborators: M. Ortner and R. Fraisse (AIRBUS Defense and Space SAS)
The goal of this large-scale project is to develop statistical and deep-learning methods for image restoration in night conditions and motion saliency detection in image sequences, respectively. The resulting algorithms will be embedded in hardware platforms for a next generation of observation satellites.
This project funded by Bpifrance concerns the PhD theses carried out by Sébastien Herbreteau and Etienne Meunier, the engineer position of Emmanuel Moebel (2022-2023), and the internships of M. Sanchez Laguardia, S. Khairi, and M. Risset.
10 Partnerships and cooperations
10.1 International initiatives
10.1.1 Participation in other International Programs
Informal international partners
Participants: Anaïs Badoual, Charles Kervrann, Ludger Johannes, Estelle Dransart, Christian Wunder, Massiullah Shafaq-Zadah.
-
–
Collaboration with Kyoto University Graduate School of Medicine (M. Arizono), Kyoto, Japan: analysis of astrocytic calcium activity. (with A. Badoual)
-
–
Collaboration with the Institute of Hydrobiology (C.A. Valades-Cruz), Chinese Academy of Sciences, Wuhan, China: Development of robust mitochondria fluorescent probes and validation in LLSM, STED and LiveSR microscopy for Live cells. (with C. Kervrann, A. Badoual)
-
–
Collaboration with the Marine Biological Laboratory (L. Leconte), Woods Hole, MA, USA : Development of robust mitochondria fluorescent probes and validation in LLSM, STED and LiveSR microscopy for Live cells. (with C. Kervrann, A. Badoual)
-
–
Collaboration with University of Campinas - UNICAMP (A. M. Dos Santos), Campinas, São Paulo State, Brazil: Novel spectral confocal imaging and super-resolution microscopy studies of membrane organization in clathrin-independent endocytosis processes with Galectin3. (with C.V. Rimoli, L. Johannes, E. Dransart, M. Shafaq-Zadah, C. Wunder)
-
–
Collaboration with NCI-NIH Bethesda (R. Weigert), USA: EGF-induced desialylation for the fast control of endocytosis. (with L. Johannes, M. Shafaq-Zadah, C. Wunder, E. Dransart)
-
–
Collaboration with Samuel Lunenfeld Research Institute (J.W. Dennis), Toronto, Canada: SLC3A2 N-glycosylation and Golgi remodeling regulate SLC7A amino acid exchangers and stress mitigation. (with L. Johannes, M. Shafaq-Zadah, C. Wunder, E. Dransart)
-
–
Collaboration with University of Copenhagen, Department of Cellular and Molecular Medicine (H. Clausen), Denmark: EGF-induced desialylation for the fast control of endocytosis; EGF-induced desialylation for the fast control of endocytosis. (with L. Johannes, M. Shafaq-Zadah, C. Wunder, E. Dransart)
-
–
Collaboration with National Centre for Biological Sciences (S. Mayor), Bangalore, India: EGF-induced desialylation for the fast control of endocytosis. (with L. Johannes, M. Shafaq-Zadah, C. Wunder, E. Dransart)
-
–
Collaboration with Lund University, Division of Microbiology, Immunology and Glycobiology (H. Leffler), Sweden: Spatial N-glycan rearrangement on α5β1integrin nucleates galectin-3 oligomers to determine endocytic fate; Endocytic roles of glycans on proteins and lipids; EGF-induced desialylation for the fast control of endocytosis. (with L. Johannes, M. Shafaq-Zadah, C. Wunder, E. Dransart)
-
–
Collaboration with Leibniz-Forschungsinstitut für Molekulare Pharmakologie (D. Roderer, S. Raunser), Berlin, Germany: Spatial N-glycan rearrangement on α5β1integrin nucleates galectin-3 oligomers to determine endocytic fate. (with L. Johannes, M. Shafaq-Zadah, C. Wunder, E. Dransart)
-
–
Collaboration with University of Namur, Department of Biology-Faculty of Sciences (H.-F. Renard), Belgium: N-BAR and F-BAR proteins - endophilin-A3 and PSTPIP1 - control the clathrin-independent endocytosis of L1CAM. (with L. Johannes, M. Shafaq-Zadah, C. Wunder, E. Dransart)
10.2 European initiatives
10.2.1 Other european programs/initiatives
ESFRI initiative program: EuroBioImaging
Participants: Charles Kervrann, Arthur Masson.
Coordinator: J. Eriksson (Turku University, Finland)
Funding: Member states of the European Union
Partners: 18 European countries in 2022 (+1 observer)
As a member of the National Research Infrastructures (RI) France BioImaging, SAIRPICO is involved in the ESFRI Euro-BioImaging project, and now in the ERIC EuroBioImaging (since November 2019), one of the landmarks of biomedical science Research Infrastructures in the roadmap of the European Strategic Forum on Research Infrastructures (ESFRI 2018). The mission of Euro-BioImaging is to provide access, service and training to state-of-the-art imaging technologies and foster the cooperation and networking at the European level including multidisciplinary scientists, industry, regional, national and European authorities.
10.3 National initiatives
10.3.1 France-BioImaging project
Participants: Charles Kervrann, Arthur Masson.
Duration: 2011 – 2024
Funding: Investissement d'Avenir, ANR INBS-PIA1 2011 and “FBI Next Generation” (ANR program 2020-2024)
Coordinator: E. Bertrand (UMR9002 CNRS) and R.-M. Mège (Institut Jacques Monod, CNRS)
Partners: CNRS, Aix-Marseille Université, Collège de France, Ecole Normale Supérieure, Ecole Polytechnique, Inria, Institut Curie, Institut Pasteur, Inserm, Université de Bordeaux, Université de Montpellier, Université de Nantes, Université de Paris, Université de Rennes, Université de Rouen, Université de Strasbourg, Université de Lyon, Université de Grenoble, Université de Toulouse.
SAIRPICO (previously SERPICO 2010-2023) is member of the French initiative (since 2011), the so-called “France-BioImaging” (FBI) National Research Infrastructure which gathers several outstanding cellular imaging centers (microscopy, spectroscopy, probe engineering and signal processing). FBI is on the French Roadmap of Research Infrastructure. The mission of FBI is to build a distributed coordinated French infrastructure for photonic and electronic cellular bioimaging, dedicated to innovation, training and technology transfer. High-computing capacities are needed to exhaustively analyze image flows.
SAIRPICO is head of the IPDM (Image Processing and Data Management) node of the FBI network composed of 11 nodes since Jan 2024. In this context, we address the following scientific problems: i/ exhaustive analysis of bioimaging data sets; ii/ deciphering of key steps of biological mechanisms at organ, tissular, cellular and molecular levels through the systematic use of time-lapse 3D microscopy and image processing methods; iii/ storage and indexing of extracted and associated data and metadata through an intelligent data management system. The team recruited R&D engineers to disseminate image processing software for large scale computing and data sets processing.
10.3.2 ANR INCREASED project: Artificial intelligence for the detection of chromosomal aberrations in dosimetry
Participants: Quentin Tallon, Charles Kervrann.
Duration: 36 months (Oct 2020 – Dec 2024)
Funding: ANR (Agence Nationale de la Recherche) ASTRID
Coordinator: Gaetan Gruel (Institut de Radioprotection et de Sureté Nucléaire (IRSN/LRAcc), Fontenay-aux-Roses)
Partners: IRSN/PSE-SANTE/SERAMED/LRAcc, SAIRPICO Team, IRBA (Institut de Recherche Biomédicale des Armées)
During insidious scenarios, when the assessment of ionizing radiation exposure condition is difficult or impossible, the quantification, on samples taken from the victims, of radio-induced chemical or biological effects is more suitable for an individualized dose reconstruction compared to theoretical calculation or Monte-Carlo simulations in regard to their great uncertainties. The INCREASED project aims to adapt the most powerful algorithms of modern artificial intelligence to the context of automatic detection of chromosomal aberrations in dosimetry. This project proposes to revisit the semi-automatic or automatic detection methods currently used for the recognition of dicentrics in GIEMSA imagery in the light of the most recent advances in artificial intelligence and deep-learning. These modern methods, which demonstrated their indisputable superiority in other areas of computer vision, will be deployed on GIEMSA images for an exhaustive multi-class count not only of dicentric chromosomes but also of ring-centric aberrations, acentric fragments, and even tricentrics. Furthermore, the INCREASED initiative is also quite innovative in dosimetry based on FISH imaging. To date, this type of dosimetric reconstruction is based on manual counting of non-exhaustive and very simplified annotations of the different possible forms of translocations. This protocol is therefore as heavy as imprecise. The INCREASED project addresses two major improvements: i/ design of a universal, rigorous and exhaustive scoring of the different forms of observable FISH translocations (3 colors). ii/ development of modern artificial intelligence algorithms able to detect and translate co-locations/co-neighborhoods into different categories of translocations.
This project concerned the PhD position of Quentin Tallon (supervised by C. Kervrann and co-supervised by M. Benadjaoud (IRSN/PSE-SANTE/SERAMED/LRAcc)) in 2024.
10.3.3 ANR POLARISCOPIA project: Next generation information processing of microscopy vector-valued images : application in cell polarized imaging
Participants: Charles Kervrann, Vincent Briane, Chencheng Gu, Leo Maury, Ferdinand Plesse–Costa.
Duration: 48 months (Oct 2022 – Sept 2026)
Funding: ANR (Agence Nationale de la Recherche) PRME
Coordinator: Charles Kervrann
Collaborators: U1143/UMR3666 (L. Johannes) and UMR168 (B. Hajj)
The objective of the project is to create the next generation of information processing techniques required to overcome the three aforementioned barriers, and to solve challenging image processing problems induced by the acquisition of 3D+Time vector-valued images. This will be achieved here by integrating concepts in statistical signal-image processing and machine learning, combined with innovative developments in fluorescence microscopy. The resulting algorithms will serve to characterize the dynamics of biomolecules and to decipher the molecular transport pathways, which are of considerable of interest in fundamental cell biology and for future precision medicine.
This project concerned the postdoc position of Vincent Briane and the PhD positions of Léo Maury and Chencheng Gu ((supervised by C. Kervrann) in 2024.
10.3.4 ANR DEEPNER project: Deciphering chromatin rearrangements in response to UV irradiation using new deep learning based cryo-electron tomography data analysis tools
Participants: Charles Kervrann, Mounir Messaoudi.
Duration: 48 months (Oct 2023 – Sept 2027)
Funding: ANR (Agence Nationale de la Recherche) PRC
Coordinator: Mikhail Eltsov (IGBMC, Strasboug)
Partners: Sorbonne University (IMPMC), Inria Rennes (SAIRPICO Team)
The goal is to reveal, with unprecedented resolution, chromatin reorganization during genotoxic stress. We will analyse the following three structural levels of the chromatin: (1) spatial organization of chromatin domains (nucleosome distribution, density, local order); (2) geometry of DNA linkers and (3) conformation and disassembly of nucleosomes. This analysis will reveal the chromatin structure-based mechanisms enabling detection and repair of UV-induced lesions in the chromatin context. To enable this analysis, we will develop appropriate DL approaches and software, in particular those for in situ cryo-ET data annotation and analysis. The added value of this research will be cryo-ET data analysis methods and algorithms embedded in software enabling not only an automated in situ annotation and analysis of nucleosomes but also other molecular complexes, in health and disease.
This project concerned the PhD position of Mounir Messaoudi ((supervised by C. Kervrann) in 2024.
10.3.5 Labex Cell(n)Scale PoMIMO – Polarization Microscopy for Imaging of Membrane Organization
Participants: Charles Kervrann, Ludger Johannes, Caio Vaz-Rimoli.
Duration: 36 months (2022 – 2024).
Funding: Labex Cell(n)Scale
Coordinator: J. Salamero.
Partners: U1143/UMR3666 (L. Johannes) and UMR168 (P. Bassereau), Institut Curie, and GATACA Systems company.
The main objective is to study the molecular organization of cargo proteins and membrane lipids by adding information on their dipole orientations. To do so, we develop innovative polarization modalities adapted to available microscopic setups and appropriate computational methods. We plan to use chemical engineering of lipid and protein-based fluorescent labels to make them rigid for polarization imaging. We aim to create a new imaging approach that will not only provide novel information about endocytosis, but will have the potential to be applied to all topics where the spatial and molecular organization of lipids/proteins defines both the structure and function of these assemblies (i.e., mitochondria cristae, MAMs....) in reconstituted systems and in living cells.
10.3.6 CNRS Project 80|Prime ModSpatioTempCell: Spatiotemporal stochastic models for the comprehension of membrane trafficking at the single cell scale
Participants: Lisa Balsolier, Frédéric Lavancier, Charles Kervrann.
Duration: 36 months (Oct 2021 – Sep 2024).
Funding: CNRS
Coordinator: F. Lavancier.
Partners: SAIRPICO Team, UMR144 Institut Curie, Laboratoire de Mathématiques Jean-Leray Nantes, UMR6629.
Endocytosis and exocytosis mechanisms involve multiple molecular components that interact through space and time according to a complex process. In this project, we plan to analyze the spatiotemporal activity of some of these proteins thanks to a tailored mathematical model, fitted to sequences of real data acquired by fluorescence microscopy. The mathematical model at hand characterizes the dynamics of a system of interacting particles with possible appearance and disappearance of some particles through time, in accordance with the observed dynamics of proteins in cell membrane trafficking. (in collaboration wit J. Salamero, CNRS-UMR144, Institut Curie)
This project concerned the PhD position of Lisa Balsollier (supervised by Frédéric Lavancier (ENSAI) and co-supervised by C. Kervrann and J. Salamero (CNRS-UMR144, Institut Curie) in 2024.
10.3.7 INRAE-Inria call - Early prediction of the transferability of bovine embryos from video-microscopy
Participant: Patrick Bouthemy.
Duration: 36 months (2023 – 2025).
Funding: INRIA
Coordinator: E. Fromont (LACODAM team, IRISA, Rennes)
Partners: UMR BREED (Ecole Nationale Vétérinaire d’Alfort), LACODAM Team
This project is concerned with the development of deep learning methods for the supervised comparison and classification of videos of in vitro fertilized (IVF) bovine embryos. The biological objective is to improve our knowledge of early phenotype formation, with ultimate applications in cattle breeding, where the challenge is to make early decision on embryo transferability. The research will also encompass the recognition of specific events during embryonic development as cell division. Challenging issues are diverse. The videomicroscopy data have specific and complex characteristics, the volume of available annotated data is limited, and the targeted classification is not that obvious.
This project involves the PhD thesis of Yasmine Hachani (supervised by E. Fromont (Lacodam Team, IRISA, Rennes) and Patrick Bouthemy) in 2024.
10.3.8 INRAE-Inria call - MreB: Role of membrane microdomains in bacterial morphogenesis
Participants: Charles Kervrann, Chencheng Gu.
Duration: 36 months (Oct 2020 – Sep 2024)
Funding: INRAE
Coordinator: R. Carballido-Lopez (INRAE UMR MICALIS, Jouy-en-Josas)
Partners: INRAE UMR MICALIS (Jouy-en-Josas), SAIRPICO Team
This project concerns the PhD thesis carried out by Claire-Jing Rouchet (since October 2020 in the INRAE UMR MICALIS, Jouy-en-Josas). The goal is to understand how dynamic molecular interactions are regulated in time and in space to form functional machineries that establish long-range orders and cellular functions. To this end we combine cutting-edge high-resolution fluorescence microscopy and spectroscopy techniques with powerful genetic, biophysical, biochemical and systems biology approaches available in model bacteria, in particular the rod-shaped Gram-positive bacterium Bacillus subtilis, to determine mechanistic details underlying basic cellular and developmental processes: the molecules, macromolecular assemblies, cellular events and physiological adaptations involved. Understanding how bacterial cells grow has important implications for the identification of new antimicrobial targets and for the development of synthetic biology tools for bioproduction and biotechnology purposes.
This project concerned the PhD position of Claire-Jing Rouchet (supervised by R. Carballido-Lopez and C. Billaudeau (INRAE UMR MICALIS) and co-supervised by C. Kervrann) in 2024.
10.4 Regional initiatives
10.4.1 Deep-SuN: Localization of chromosomal aberrations induced by nuclear radiation dose excess
Participant: Charles Kervrann.
Funding: IRSN (Institut de Radioprotection et Sureté Nucléaire), Région-Bretagne
Duration: 48 months (Oct 2020 – Sep 2024)
This project concerned the PhD thesis carried out by Antonin Deschemps in 2023 (supervised by C. Kervrann and co-superivsed by M. Benadjaoud (IRSN)), collaboration with IRSN/PSE-SANTE/SERAMED/LRAcc (see DeepSun projet - Section 9.1.1).
11 Dissemination
11.1 Promoting scientific activities
11.1.1 Scientific events: selection
Participants: Charles Kervrann.
Member of the conference program committees
-
–
Charles Kervrann was member of the scientific committee of IABM'2024 (Colloque Français d'Intelligence Artificielle en Imagerie Biomédicale (IABM)).
11.1.2 Journal
Participants: Charles Kervrann, Ludger Johannes, Anaïs Badoual, Frédéric Lavancier.
Member of the editorial boards
-
–
Charles Kervrann is Associate Editor for the "IEEE Transactions on Image Processing" (since 2021) and "Biological Imaging" (since 2021) jounals .
-
–
Ludger Johannes is member of the Editorial Board of the "Biology of the Cell" journal (since 2010) and "Toxins" (since 2015), and Academic Editor for the "PLoS ONE" journal (since 2013).
-
–
Frédéric Lavancier is Associate Editor for the "Scandinavian Journal of Statistics” (since 2024) and for “Stateco” (since 2023).
Reviewer - reviewing activities
-
–
Charles Kervrann was reviewer for "Int. J. Computer Vision" and "IEEE Transactions on Image Processing" in 2024.
-
–
Ludger Johannes was reviewer for Nature, Nat Commun, Science Adv, Cell Reports, PNAS, J Cell Biol, Biol Cell, PLoS One, Frontiers, EMBO J, Biochem Soc Trans, Science, Biochem Cell Biol, and ACS Nano in 2024.
-
–
Anaïs Badoual was reviewer for "Nature Methods" in 2024.
-
–
Frédéric Lavancier was reviewer for "Applied Probability" journals in 2024.
11.1.3 Invited talks
Participants: Charles Kervrann, Ludger Johannes, Anaïs Badoual, Frédéric Lavancier, Arthur Masson.
-
–
Charles Kervrann:
-
>"Computational and quantitative bioimaging for deciphering intracellular dynamics and structure at the cell scale", FACULTY Meeting of the Research Center of Institut Curie, Paris, France, February
-
>"Biological imaging and computational microscopy", PIANOFORTE workshop on "Artificial Intelligence and Radiation Protection", Athens, Greece, April.
-
>"Méthodes statistiques et d'intelligence artificielle appliquées aux images de microscopie pour sonder l'intérieur des cellules à l'échelle nanométrique", Journées des Neurosciences Translationnelles NeurATRIS, Pornichet, France, May.
-
>"Statistical and artificial intelligence methods for live-cell fluorescence imaging and cryo-electron tomography", ITMO-BCDE workshop on "AI & imaging for Cell and Development Biology: Insights, Limits, and Perspectives ", Paris, France, September.
-
>"IPDM - BioImage Informatics Node: results, perspectives, and roadmap", France BioImaging Annual Meeting, Strasbourg, France, November.
-
>"Statistical and artificial intelligence methods for live-cell fluorescence imaging and cryo-electron tomography", Institut de Biologie Structurale, Grenoble, France, October. (seminar)
-
>"Statistical and artificial intelligence methods for live-cell fluorescence imaging and cryo-electron tomography", IMT Atlantique, Brest, France, March. (seminar)
-
>
-
–
Ludger Johannes:
-
>Desialylation controls glycolipid-lectin driven formation of clathrin-independent carriers", EMBO workshop - Trafficking and Glycosylation at the Golgi apparatus, Sorrento, Italy, April.
-
>"GlycoSwitch: a novel signaling circuit to control endocytosis", Harvard Digestive Diseases Center (HDDC) Spring Symposium, Boston, USA, June.
-
>"Shiga toxin-based targeting of dendritic cells for vaccination", 8th Barrande Bioscience Meeting, Paris, France, October.
-
>"Chemical Biology between France and China", 2024 China-Europe Scientists Forum, Frankfurt am Main, Germany, October.
-
>"The sugar path to biomedical discovery", 3rd International Symposium on Clinica Medicine and Intelligent Devices (CMID2024), Frankfurt am Main (Germany), October. (Keynote lecture)
-
>"GlycoSwitch: a novel signaling circuit to control endocytosis", Meeting - Organelles Transport in Health and Disease, Grenoble, France, December.
-
>"Glycosylation in endocytosis and therapeutic intracellular delivery", European Institute of Chemistry and Biology, Bordeaux, France, March. (seminar)
-
>"GlycoSwitch: a novel signaling circuit to control endocytosis". National Institutes of Health, Bethesda, USA, May. (seminar)
-
>"GlycoSwitch: a novel signaling circuit to control endocytosis", University of Virginia, Center for Membrane and Cell Physiology, Charlottesville, USA, June. (seminar)
-
>"Glycoswitches for the control of endocytosis and retrograde trafficking", Institut Pasteur, Hong-Kong, China, July. (seminar)
-
>
-
–
Anaïs Badoual:
-
>"Hybrid CNN-Snake algorithms for the segmentation of 3D microscopy images", Numerical Analysis & Modelling in Applies Sciences (NAMAS), Gaeta, Italy, September.
-
>"Geometric representations and algorithms for the quantitative analysis of 3D live cell images", IstroSight team, Lyon, France, May. ((seminar)
-
>"DeepCristae, a CNN for the restoration of mitochondria cristae in live microscopy images", Chemical Biology for Cancer (CBC), Institut Curie, Paris, France, April. (seminar)
-
>
-
–
Frédéric Lavancier:
-
>“Spatial birth-death-move processes : basic properties and inference”, University of Gothenburg, Sweden, January. (seminar)
-
>"Non-parametric intensity estimation of spatial point processes by random forests”, Journées de Statistiques (JDS), Bordeaux, France, May. (plenary talk)
-
>
-
–
Arthur Masson:
-
>"BioImageIT : open-source solution unifying data management and image analysis", Chemical Biology for Cancer (CBC), Institut Curie, Paris, France, June. (seminar)
-
>"BioImageIT : open-source solution unifying data management and image analysis", FBI-IPDM Node Meeting, Insitut Pasteur, Paris, France November. (demonstration)
-
>
11.1.4 Leadership within the scientific community
Participants: Charles Kervrann, Ludger Johannes.
-
–
Charles Kervrann is head of the "BioImage Informatics" node (ANR France-BioImaging project since Jan 2024, National Research Infrastructure" for Biology and Health) (co-head since 2011). He is member of the IEEE Signal Processing Society (since 2010).
-
–
Ludger Johannes is member of the American Society for Cell Biology (since 1996), Société de Biologie Cellulaire de France (since 1996), French Society for Biophysics (SFB) and Membrane Study Group (GEM) (since 2012), Société de Chimie Thérapeutique (SCT) (since 2014), Biophysical Society (since 2015), Société Française pour l'Etude des Toxines (SFET) (since 2021), Société Française du Cancer (SFC) (since 2022), Société Chimique de France (SCF) (since 2023).
11.1.5 Scientific expertise
Participants: Charles Kervrann, Ludger Johannes, Patrick Bouthemy, Frédéric Lavancier, Christian Wunder.
-
–
Charles Kervrann was member of the selection committee for the recruitment of a Professor (University of Strasbourg, CNU 61) and of a Junior Professor Chair (Mines Paris Université PSL, Paris Sciences et Lettres, CNU 26-27-61).
-
–
Ludger Johannes is:
-
>Member of the scientific council of the Indo-French Centre for the Promotion of Advanced Research (IFCPAR/CEFIPRA (since 2021).
-
>Member of the permanent SVE3 HCERES expert panel (independent agency for the reviewing of research structures in France) (since 2021).
-
>President of the HCERES mission UMR1279 - "Dynamiques des Cellules Tumorales", Villejuif (December 2024).
-
>member of the Scientific Council of the "Membrane Study Group"since 2021.
-
>Member of the scientific council of the Chemical Biology Interest Group (GDR Chémobiologie) since 2021.
-
>Member of the scientific committee of the Cellular and Tissular Imaging Platform (PICT) at CurieCoreTech of Institut Curie since 2021.
-
>Member of the scientific council of Doctoral School BIOSIGNE (ED568) since 2014.
-
>Scientific expert for the reviewing of projects for the European Innovation Council (EIC), European Research Council (ERC), Cancéropôle PACA, HCERES, CEFIPRA, Fondation Maladies Rares, Israel Science Foundation, and ANR in 2024.
-
>
-
–
Patrick Bouthemy was head of the HCERES committee that evaluated the LISIC (Laboratoire d'Informatique, Signal et Image de la Côte d'Opale).
-
–
Christian Wunder was Vice-chair for Evaluation of Marie Sklodowska-Curie Actions (MSCA) in HorizonEurope.
-
–
Frédéric Lavancier was scientific expert for the reviewing of projects from the Swiss National Science Foundation.
11.1.6 Research administration
Participants: Charles Kervrann, Patrick Bouthemy, Ludger Johannes.
-
–
Charles Kervrann:
-
>Head of the "BioImage Informatics" node (ANR France-BioImaging project, National Research Infrastructure" for Biology and Health) since Jan 2024 (co-head since 2011).
-
>
-
–
Ludger Johannes:
-
>Head of Traffic, Signaling and Delivery team at Curie Institute since 2001.
-
>Director of Cellular and Chemical Biology unit (U1143 INSERM, UMR3666 CNRS) since 2014.
-
>Scientific director of the Metabolomics and Lipidomics Platform at CurieCoreTech of Institut Curie since 2021.
-
>Representative of Doctoral School BIOSIGNE (ED568) in the Doctoral College of PSL University since 2016.
-
>Institut Curie coordinator of institutional partnership between Institut Curie, CNRS and the National Centre for Biological Sciences (NCBS) à Bangalore (India) since 2012.
-
>
-
–
Patrick Bouthemy:
-
>Member of the executive board of Excellence Lab (Labex) CominLabs.
-
>Member of the Selection and Validation Committee of the Images & Réseaux competitivity cluster.
-
>Member of the steering committee of the medical imaging Neurinfo platform.
-
>
11.2 Teaching - Supervision - Juries
11.2.1 Teaching
Participants: Charles Kervrann, Anaïs Badoual, Ludger Johannes, Lisa Balsollier, Quentin Rapilly.
-
–
Charles Kervrann:
-
>Master 2: "From Bioimage Processing to BioImage Informatics", 3 hours (4.5 hours TD), coordinator of the module (30 hours / equiv 45 hours TD), Master 2 Research IRIV, Telecom-Physique Strasbourg and University of Strasbourg.
-
>
-
–
Ludger Johannes
-
>Master 2: "Endocytic trafficking" for "Chemical Frontiers of Living Cells", 2 hours (3 hours TD), PSL Research University.
-
>"Cell biology" course, 1 week, Institut Pasteur and Hong-Kong University (China).
-
>Lecture "Molecular Biology of the Cell", 2 hours, Institut Pasteur/Curie, lectures and lab training.
-
>
-
–
Anaïs Badoual:
-
>Master degree: "Analysis of Image Sequences", 9 hours (13.5 hours TD) and 3 hours Practical Courses (2 hours TD), Master 2 Research SiVos, ISTIC & University of Rennes.
-
>Master degree: "Object Tracking in microscopy", 1.75 hours (2.63 hours TD), Master 2 Research IRIV, Telecom-Physique Strasbourg.
-
>
-
–
Lisa Balsollier:
-
>Licence (L1) degree : "Mathematics for biology", 30 hours TD, University of Rennes 1.
-
>Master degree : "Probabilities and statistics (for "agrégation interne"), 3 hours (4,5 hours TD) and 28 hours TD, University of Rennes.
-
>
-
–
Quentin Rapilly:
-
>Licence (L2) degree: "Introduction to probabilities theory", 20 hours (TD), INSA Rennes
-
>Licence (L1) degree: "Introduction to mathematics for engineering", 16 hours (Cours/TD), INSA Rennes/ENSAB
-
>
-
–
Frédéric Lavancier:
-
>Engineer degree (2nd year) : “Generalized and linear regression models”, 24 hours (36 hours TD), coordinator, ENSAI.
-
>Engineer degree (2nd year) : “Markovian models”, 21 hours (31.5 hours TD), coordinator, ENSAI.
-
>Doctoral course : “A short introduction to models and inference for spatial point processes”, 4 hours, ED Mathstic Rennes.
-
>
11.2.2 Supervision of students in team
Participants: Charles Kervrann, Anaïs Badoual, Ludger Johannes, Massiullah Shafaq-Zadah, Estelle Dransart, Frédéric Lavancier.
-
–
PhD supervision
-
>Quentin Tallon (PhD defended in January 2025, AID/DGA grant): "Statistical learning for localization and classification of chromosomal aberrations in FISH microscopy images" (started in October 2021, supervised by C. Kervrann and M.A. Benadjaoud (IRSN/SERAMED, Fontenay-aux-Roses)).
-
>Lisa Balsollier (PhD defended in October 2024, CNRS grant): "Spatiotemporal stochastic models for the comprehension of membrane trafficking at the single cell scale" (started in October 2021, co-supervised by F. Lavancier, C. Kervrann, and J. Salamero). (tel-04881209)
-
>Quentin Rapilly (PhD in progress, Inria grant): "Hybrid CNN-Snake algorithms for quantitative analysis of 3D+time live-cell images" (started in December 2022, supervised by A. Badoual and C. Kervrann).
-
>Léo Maury (PhD in progress, ANR Polariscaopia grant): "Machine learning and optimization methods for 3D vector-valued microscopy image reconstruction" (started in January 2024, supervised by C. Kervrann).
-
>Chencheng Gu (PhD in progress, ANR Polariscaopia grant): "Spatial statistics and machine learning for molecular dynamics analysis in polarized microscopy" (started in March 2024, supervised by C. Kervrann).
-
>Mounir Messaoudi (PhD in progress, ANR DeepNer grant): "Machine learning for 3D cryo-electron tomogram analysis: localization, identification, and spatial organization of macromolecules in cells" (started in May 2024, supervised by C. Kervrann).
-
>Ferdinand Plesse–Costa (PhD in progress, Inria grant): "Statistical and machine learning for image reconstruction and super-resolution in fluorescence polarized microscopy" (started in November 2024, co-supervised by C. Kervrann, B. Hajj (UMR168-Institut Curie), and C. Vaz-Rimoli).
-
>
-
–
Postdoc supervision
-
>Vincent Briane (ANR Polariscopia grant), since March 2023, supervised C. Kervrann.
-
>Ilyes Hamitouche (Inserm gant), since June 2023, co-supervised by M. Shafaq-Zadah, E. Dransart, C. Kervrann, and L. Johannes.
-
>
-
–
Master supervision
-
>Theotim Barbier, INSA Rouen, Master 1, June-August 2024, supervised by C. Kervrann.
-
>Ferdinand Plesse–Costa, University Paris-Saclay, Master 2 ATSI, March-August 2024, supervised by C. Kervrann.
-
>Gabriel Nampoina Ravalomanana, University Paris Dauphine-PSL, Master 2 IASD, April-August 2024, co-supervised by C. Kervrann and T. Pécot (University of Rennes).
-
>
11.2.3 Juries
Participants: Charles Kervrann, Ludger Johannes, Frédéric Lavancier.
-
–
Charles Kervrann:
-
>President of the PhD thesis committee of :
- M. Ounissi - Decoding the black box: enhancing interpretability and trust in AI for biomedical imaging - A step toward responsible artificial intelligence. Sorbonne University, supervised by D. Racoceanu.
-
>Reviewer of the HdR of :
- S. Ladjal - Télécom Paris, Institut Polytechnique de Paris. (defense in September 2024)
- M. El Beheiry - Towards a unified framework for multidimensional scientific data interpretation. University Paris-Saclay. (defense in February 2024)
- A. Chessel - On advancing biology with images. Ecole Polytechnique, Institut Polytechnique de Paris. (defense in January 2024)
- T. Lagache - Statistical methods and stochastic modeling in biological imaging. Institut Pasteur, University Paris Cité. (defense in February 2024)
-
>Reviewer for the PhD theses of :
- T. Eloy - Reconstruction à partir de particule isolée en microscopie de fluorescence. Unviversity of Strasbourg, supervised by E. Baudrier and D. Fortun. (defense in September 2024)
- N. Quiblier - Intracellular signaling in spatially heterogeneous media: modelling and statistical learning methods. University Claude bernard Lyon 1, supervised by H. Berry. (defense in December 2024)
- L. Gaifas - Enabling superstructural studies of biological assemblies by CryoET. Institut de Biologie Structurale, University of Grenoble Alpes, supervised by I. Gutsche. (defense in October 2024)
- A. Artola - A generic method for detecting anomalies on manufactured parts. Ecole Normale Supérieure Paris-Saclay, University Paris-Saclay, supervised by J.M. Morel and T. Ehret. (defense in June 2024)
-
>Member of the HdR thesis committee of :
- P. Arias - Video restoration: from patch-based methods to self-supervised learning. Ecole Normale Supérieure Paris-Saclay. (defense in December 2024)
-
>Member of the PhD thesis committee of :
- C.J. Rouchet - Effect of membrane fluidity on cell wall shape and elongation in Bacillus subtilis. Institut MICALIS-INRAE, University of Paris Cité, supervised by R. Carballido-López, C. Billaudeau, and C. Kervrann. (defense in November 2024)
- L. Balsollier - Simulation, parametric estimation, and trajectory classification of a birth-death-move process with mutations. Laboratoire de Mathématiques Jean Leray, University of Nantes, supervised by F. Lavancier, J. Salamero, and C. Kervrann. (defense in October 2024)
-
>
-
–
Ludger Johannes:
-
>Reviewer of the PhD thesis of :
- Alexandre Wolf - Mise au point et utilisation d'une boite à outils moléculaires pour étudier les fonctions pléïotropiques de l'acide phosphatidique au cours de la neurosecretion, supervised by N. Vitale, University of Strasbourg. (Defense in September 2024)
- Parisa Khalilian - Le rôle du trafic intracellulaire des protéines associées à la membrane dans la polarité épithéliale et le cancer, supervised by S. Lebreton, Institut Pasteur. (Defense in September 2024)
-
>Member of the PhD thesis committee of:
- Shiqiang Xu - Clathrin-independent endocytosis and retrograde transport in cancer cells promote cytotoxic CD8 T cell activation, supervised by H.-F. Renard, Université de Namur, Belgium, (Defense in December 2024)
-
>
-
–
Frédéric Lavancier:
-
>Reviewer of the PhD thesis of :
- Bartholomé Vieille - Caractérisation de la distribution de Processus ponctuels marqués auto-excitants de Hawkes et de Markov. INRAE-BioSP Unit, University of Aix Marseille, supervised by S. Soubeyrand and R. Senoussi. (defense in June 2024)
-
>Reviewer of the HdR of :
- Simon Barthelmé - Almost exclusively linear algebra : an introduction to determinantal point processes. Universitéy of Grenoble Alpes. (defense in september 2024)
-
>
12 Scientific production
12.1 Major publications
- 1 articleCeramide structure dictates glycosphingolipid nanodomain assembly and function.Nature Communications121December 2021, 3675HALDOI
- 2 articleStatistical analysis of particle trajectories in living cells.Physical Review E 976June 2018, 1-20HALDOI
- 3 articleFunctional dissection of the retrograde Shiga toxin trafficking inhibitor Retro-2.Nature Chemical Biology163March 2020, 327-336HALDOI
- 4 articleDCT2net: an interpretable shallow CNN for image denoising.IEEE Transactions on Image Processing31June 2022, 4292 - 4305HALDOI
- 5 articleTesting independence between two random sets for the analysis of colocalization in bioimaging.Biometrics761March 2020, 36-46HALDOI
- 6 articleDeep Learning Improves Macromolecule Identification in 3D Cellular Cryo-Electron Tomograms.Nature Methods1811October 2021, 1386-1394HALDOI
- 7 articleBioImageIT: Open-source framework for integration of image data-management with analysis.Nature Methods19November 2022, 1328–1330HALDOI
12.2 Publications of the year
International journals
International peer-reviewed conferences
National peer-reviewed Conferences
Conferences without proceedings
Doctoral dissertations and habilitation theses
Reports & preprints
Other scientific publications
Scientific popularization

