Section: Research Program
Excitable systems: analysis of physiological time series
The research described in this section is a collaboration effort of GEOSTAT, CNRS LOMA (Laboratoire Ondes et Matière d'Aquitaine) and Laboratory of Physical Foundation of Strength, Institute of Continuous Media Mechanics (Perm, Russia Federation).
|
AF is an arrhythmia originating in the rapid and irregular electrical activity of the atria (the heart’s two upper chambers) that causes their pump function to fail, increasing up to fivefold the risk of embolic stroke. The prevailing electrophysiological concepts describing tachy-arrhythmias are more than a century old. They involve abnormal automaticity and conduction [53]. Initiation and maintenance are thought to arise from a vulnerable substrate prone to the emergence of multiple self-perpetuating reentry circuits, also called “multiple wavelets” [58], [59]. Reentries may be driven structurally, for instance because of locally high fibrous tissue content which badly conducts, or functionally because of high spatial dispersion of decreased refractoriness and APD [57]. The latter is coined the leading circle concept with the clinically more relevant notion of a critical “wavelength” (in fact the length) of the cardiac impulse [29], [63], [61], [33]. The related concept of vulnerability was originally introduced to uncover a physiological substrate evolving from normality to pathology. It was found in vulnerable patients that high rate frequency would invariably lead to functional disorder as cardiac cells would no longer properly adapt their refractoriness [32]. Mathematical models have managed to exhibit likewise phenomena, with the generation of breaking spiral waves in various conditions [50], [55]. The triggering role of abnormal ectopic activity of the pulmonary veins has been demonstrated on patients with paroxysmal AF resistant to drug therapy [49], but its origin still remains poorly understood. This region is highly innervated with sympathetic and parasympathetic stimulation from the ANS [65], [66], [31]. In particular, Coumel et al. [41], [40] have revealed the pathophysiological role of the vagal tone on a vulnerable substrate. It is frequently observed that rapid tachycardia of ectopic origin transits to AF. This is known to result from electrical remodeling. As described for the first time by Allessie et al. [28], remodeling is a transient and reversible process by which the impulse properties such as its refractory period are altered during the course of the arrhythmia, promoting its perpetuation: “AF begets AF” [68]. Under substantial beating rate increase, cells may undergo remodeling to overcome the toxicity of their excessive intercellular calcium loading, by a rapid down regulation (a few minutes) of their L-type calcium membrane current. Moreover, other ionic channel functions are also modified such as the potassium channel function, inducing a change in the conduction properties including the conduction velocity. The intercellular coupling at the gap junction level shows also alterations of their connexin expression and dispersion.
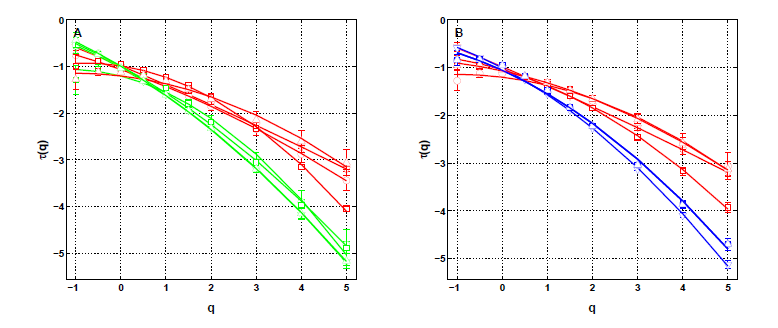
Wavelet-based methods (WTMM, log-cumulants, two point scale correlations), and confidence statistical methodology, have been applied to catheter recordings in the coronary sinus vein right next to the left atria of a small sample of patients with various conditions, and exhibit clear multifractal scaling without cross-scale correlation, which are coined ”multifractal white noise”, and that can be grouped according to two anatomical regions. One of our main result was to show that this is incompatible with the common lore for atrial fibrillation based on so-called circuit reentries. We used two declinations of a wavelet-based multi-scale method, the moment (partition function) method and the magnitude cumulant method, as originally introduced in the field of fully developed turbulence. In the context of cardiac physiology, this methodology was shown to be valuable in assessing congestive heart failure from the monitoring of sinus heart rate variability [51]. We develop a model such that the substrate function is modulated by the kinetics of conduction. A simple reversible mechanism of short term remodeling under rapid pacing is demonstrated, by which ionic overload acts locally (dynamical feedback) on the kinetics of gap junction conductance. The whole process may propagate and pervade the myocardium via electronic currents, becoming desynchronized. In a new description, we propose that circuit reentries may well exist before the onset of fibrillation, favoring onset but not contributing directly to the onset and perpetuation. By contrast, cell-to-cell coupling is considered fundamentally dynamical. The rationale stems from the observation that multifractal scaling necessitates a high number of degrees of freedom (tending to infinity with system size), which can originate in excitable systems in hyperbolic spatial coupling. See figure 1.