Keywords
Computer Science and Digital Science
- A3.1.1. Modeling, representation
- A3.3.2. Data mining
- A3.3.3. Big data analysis
- A3.4.1. Supervised learning
- A3.4.2. Unsupervised learning
- A3.4.5. Bayesian methods
- A6.1.1. Continuous Modeling (PDE, ODE)
- A9.2. Machine learning
Other Research Topics and Application Domains
- B1.1.8. Mathematical biology
- B2.2.3. Cancer
- B2.4.1. Pharmaco kinetics and dynamics
- B2.4.2. Drug resistance
1 Team members, visitors, external collaborators
Research Scientist
- Sebastien Benzekry [Team leader, Inria, Researcher, HDR]
Faculty Members
- Joseph Ciccolini [Team leader, APHM, AMU, Professor]
- Dominique Barbolosi [AMU, Professor]
- David Boulate [APHM, Hospital Staff, from May 2022, MD, PhD]
- Raphaelle Fanciullino [APHM, AMU, Associate Professor]
- Florence Gattacceca [AMU, Associate Professor]
- Laurent Greillier [APHM, AMU, Professor]
- Athanassios Iliadis [AMU]
- Xavier Muracciole [APHM, Hospital Staff]
- Anne Rodallec [AMU, Associate Professor]
- Sebastien Salas [APHM, AMU, Professor]
Post-Doctoral Fellows
- Paul Dufossé [Inria, ENSAE, L3]
- Abdessamad El Kaoutari [Inserm]
- Rossana Passanante [AMU]
PhD Students
- Celestin Bigarre [Inria]
- Mathilde Dacos [APHM]
- Anthéa Deschamps [APHM]
- Mourad Hamimed [AMU]
- Clémence Marin [AMU]
- Linh Nguyen Phuong [AMU, from Sep 2022]
- Loic Osanno [APHM]
- Jessica Ou [AMU, from Apr 2022]
- Raphael Serre [CHIU Limoges, until Jun 2022]
- Guillaume Sicard [APHM]
Technical Staff
- Sarah Giacometti [AMU, Technician]
- Melanie Karlsen [Inserm, Engineer]
- Sophie Marolleau [AMU, Engineer]
Interns and Apprentices
- Liza Al Shikhley [Inria, from Feb 2022, UTC Compiègne, M2]
- Rémy Harle [Inria, from Mar 2022 until Jun 2022, AMU, M1]
- Govind Kallee [APHM, Intern, from Sep 2022]
- Mathieu Lalaque [Inria, from Jun 2022, Centrale Marseille, M1]
- Juliette Lepère [Inria, from Jun 2022 until Aug 2022, ENSAE, L3]
- Charles Martial [AMU, from Mar 2022 until Jun 2022, M1]
- Carla Perez [AMU, from Oct 2022]
- Dorian Protzenko [APHM, Intern, from Sep 2022]
Administrative Assistant
- Sandrine Boute [Inria]
Visiting Scientist
- Laurent Bourguignon [CHU Lyon, Université Lyon 1, until Aug 2022, HDR]
2 Overall objectives
We aim to optimize therapeutic approaches (i.e., controlling toxicities while ensuring a maximal efficacy) in oncology using mechanistic and statistical modeling (see Figure 1). These therapeutic approaches include immunotherapy, radiotherapy, chemotherapy, targeted therapies and their planification: combinations, sequences, intensification – densification, dosing and scheduling. Specifically, our research will be organized along three main axes:
- Quantitative modeling for personalized clinical oncology.
- Individualizing anticancer drugs regimen.
- Optimizing combinatorial strategies with immune checkpoint inhibitors.
Of note, in the Research Priorities document released by the American Society of Clinical Oncology in February 2021, “Developing and Integrating Artificial Intelligence in Cancer Research”, “Identifying Strategies That Predict Response and Resistance to Immunotherapies” and “Optimizing Multimodality Treatment for Solid Tumors” are listed as top-priorities, which fit quite well with our 3 axes.
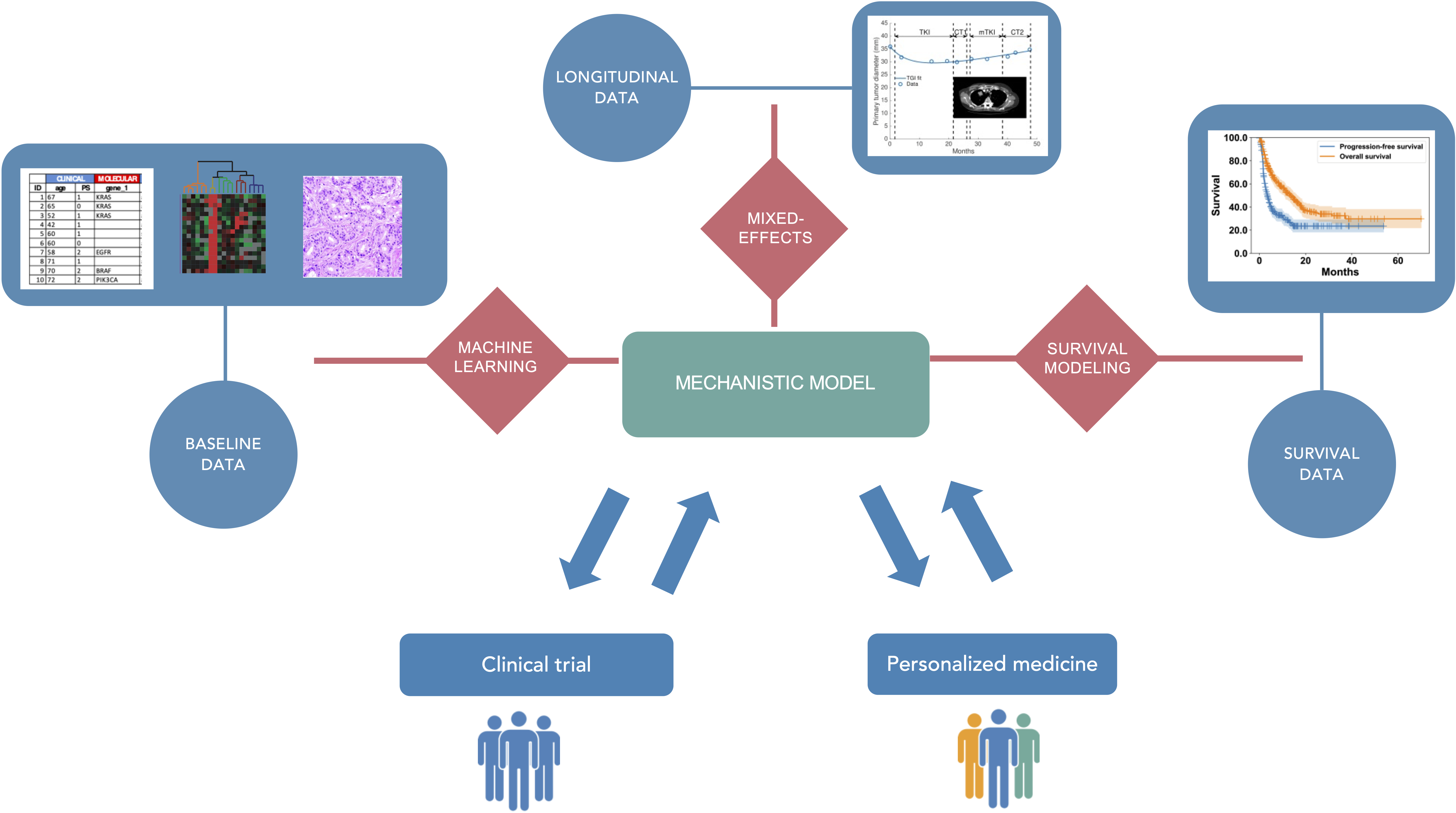
Mechanistic and statistical modeling for pharmacological and clinical oncology.
3 Research program
3.1 Scientific context and motivations
The project-team is based upon the development of model-driven clinical oncology as a means to optimize anticancer therapies. Despite continuous efforts to make available novel drugs beyond traditional cytotoxic chemotherapy (i.e., oral targeted therapies, biologics, immune checkpoint inhibitors), prognosis of outcome remains poor for many cancers. The dosing regimen of anticancer drugs given today remains largely empirical, because dose-finding studies are often performed using outdated, sub-optimal protocols (such as modified-Fibonacci dose-ranging protocols) or because concomitant administration is the rule when combining several drugs. Consequently, clinical oncologists struggle to refine the way they use the anticancer agents made at their disposal. For instance, it took several years of bedside practice to understand that paclitaxel in breast cancer patients should not be administrated using the officially approved 150 mg/m² every 3 weeks scheduling, but rather with an alternate 75 mg/m² weekly dosing 112. Similarly, multi-targets sunitinib is now given on a 50 mg two-weeks on / one-week off basis, rather than the officially approved four-weeks on / two-weeks off schedule 100. Elsewhere, several combinatorial strategies trials have failed to yield convincing results, mostly because of the lack of a strong rationale regarding the best way to sequence treatments 94. Globally, clinical oncology today is still all about finding the best way to treat patients ensuring an optimal efficacy / safety balance.
After having long been limited to cytotoxic chemotherapy (in addition to surgery and radiotherapy), the arsenal of anti-cancer agents has dramatically increased over the last two decades. Indeed, major advances in the understanding of cancer biology, including: 1) the discovery and quantification of (epi)genetic alterations leading to targeted therapy and 2) the realization of the importance of the non-cancer cell components of tumors, i.e., the tumor micro-environment and tumor immunity, have helped to identify novel targets. Drugs targeting the tumor vasculature (e.g., first-in-class bevacizumab, approved in the mid-2000’s 82) or tumor immunity (e.g., immune checkpoint inhibitors (ICI) such as first-in-class ipilimumab, approved in the early 2010’s 110) represent groundbreaking innovations in oncology. ICIs in particular are considered as game-changing drugs because diseases with once dismal prognosis (e.g., metastatic melanoma, non-small cell lung cancer (NSCLC), kidney cancer or head and neck cancer 132) now show 20-40% of 5-years survival. Nevertheless, these impressive results are limited to a minority of patients in a limited number of cancers. In addition, no validated biomarker predictive of response has yet been identified, thus highlighting how early prediction of response and probability of future relapse are a critical, unmet medical need. The encouraging yet still insufficient clinical results of ICIs have led current clinical oncology to consider combinations of such immunotherapies with preexisting anti-cancer modalities: radiation therapy 119, cytotoxic 106, targeted 124 or anti-angiogenic therapies 131. However, the near-infinite possibilities of combinations in terms of sequencing, dosing and scheduling challenge the ability of classical trial-and-error methods to find appropriate modes of combination 93.
In addition, day-to-day clinical decisions made by oncologists are based on a large amount of information, coming from: 1) their own knowledge integrating years of clinical practice combined to updated literature and 2) objective data coming from multiple sources (demographic data, radiology, functional imaging, molecular biology, histology, biomarkers, blood counts, etc…). The large amount of clinical and biological data generated now in clinical oncology is not properly analyzed, because of the lack of appropriate models to picture the complexity of longitudinal observations. Oncologists lack a comprehensive framework and numerical software that could support decision of therapeutic strategy (e.g., to treat or not? to what extent? with what treatment (surgery, radiotherapy, systemic therapy)? in what order? etc.), especially when their time dedicated to examination of a given patient case is limited (e.g., in multidisciplinary meetings (RCP), or individual consultations). Furthermore, modeling is the only way to retrieve similar characteristics from very different experimental conditions and clinical protocols.
To address these major issues, our project-team aims at:
- guiding anticancer therapy by developing patient-specific predictive models (individual level);
- better designing clinical trials, in particular regarding combinatorial trials (population level).
3.2 Data
We use non-clinical and clinical data related with the pharmacology of anti-cancer agents and medical monitoring of the disease status. The former includes pharmacokinetics (drug levels in plasma (patients) and full body pharmacokinetics (animal models)), pharmacodynamics (efficacy, safety), pharmacogenetics (i.e., constitutional genetic polymorphisms affecting drug transport and metabolizing enzymes), pharmacogenomics (i.e., molecular and genetic alterations affecting tumor cells). The latter include demographics, anatomical imaging (e.g., tumor sizes derived from CT scan or MRI), functional imaging (e.g., positron emission tomography), histopathology quantifications, biological variables (such as kidney and liver functions or blood counts), immuno-monitoring data (flow cytometry) or cell free DNA. We will especially rely on real-world data (also termed fragmentary data) collected from patient routine monitoring by our members with hospital activity.
Experimental data are generated by the experimental wet-lab group (AR, RF, JC), relying on state-of-the-art experimental pharmacokinetic laboratory fully equipped to perform in vitro and in vivo explorations of drug metabolism, pharmacokinetics and experimental therapeutics in oncology, including bioanalytical support and fluorescence/bioluminescence monitoring in rodents with highly specialized staff. Clinical pharmacokinetics and pharmacogenetics data are generated by the clinical pharmacology group (JC, RF), relying on the expertise of the clinical pharmacokinetics laboratory of the La Timone University Hospital of Marseille, an FDA-labelled, ISO15189-labelled facility with state-of-the-art bioanalytical resources to assay any kind of drugs or drug metabolites in patients. Specific data regarding cancer biology and pharmacodynamics (immunomonitoring, pharmacogenomics) are generated in collaboration with other CRCM teams.
Clinical data and additional biomarker data are collected from either clinical trials or real-world studies performed by hospital pharmacists and oncologists of the joint-team (RF, JC, SS, LG, XM) and their residents, or by other medical oncologist partners. We have strong collaborations and ongoing projects with pediatrics (Pr N. Andre), hematology (Dr G. Venton), nuclear medicine (Pr D. Taïeb) and radiotherapy (Pr L. Padovani). Importantly, the project-team is located near the INCa-labeled center for early clinical trials (CLIP2), thus facilitating the data collection and later, the implementation of modeling approaches in early clinical trials.
In addition, we also rely on publicly available data from online databases such as the TCGA (genomic data), the TCIA (imaging data) or data from clinical trials.
3.3 Mathematical methodology
Our primary objective is centered on the improvement of therapeutic strategies in oncology. Nevertheless, this brings novel methodological challenges requiring developments at the formal level within the generic field of modeling biological and pharmaco-patho-physiological systems. Difficulties to take into account include: the longitudinal profile of the quantities of interest; measurement uncertainty (requiring statistical considerations); difficulties in sampling the real processes leading to scarcity of the observed data and large inter-individual variability. Many specific problems in life science systems are very different from that encountered from physical modeling in industrial applications (e.g., mechanical engineering or energy).
To summarize our methodology, we are interested in modeling the dynamics of pharmaco-oncological processes (mechanistic modeling) and their inter-individual variability (statistical modeling). Our intended methodological contributions are: 1) to invent novel mechanistic models for complex physiological processes able to describe the effect of therapeutic intervention, 2) to design appropriate statistical frameworks for parameter estimation and description of inter-individual variability, 3) to test and validate the models against experimental and clinical data, 4) to combine state-of-the art machine learning (ML) methods with mechanistic models to integrate large dimension data.
3.3.1 Mechanistic modeling
Mechanistic models are defined here as mathematical constructs that describe physiological variables (e.g., plasma drug concentration, tumor size, or biomarkers) and their dynamics based on physical and biological principles (e.g., law of mass conservation). They describe the time profiles of the variables of interest by means of ordinary or partial differential equations (ODEs and PDEs, respectively) and are thus deterministic.
The main challenge of the modeling exercise is to find the appropriate balance between the degree of integration of biological phenomena (model complexity) and granularity of the data available (i.e., sampling time resolution, observed variables, spatio-temporal or only temporal measurements) ensuring the feasibility of parameter estimation. Indeed, cancer biology is extremely complex, involving processes at multiple temporal and spatial scales (intra- and inter-cellular, tissular, organism). It is thus tempting to build intricate models integrating as many phenomena as possible. Along these lines, the last decades have witnessed the proliferation of multiple such complex models. We see two shortcomings to this approach. First, in contrast with models of physical phenomena, the parameters of biological models are often not directly measurable and thus have to be estimated from fitting the models to experimental or clinical data. Therefore, their number has to be commensurate to the available data in order to ensure identifiability. Unfortunately, many complex models from mathematical oncology have too many parameters to be reasonably identified and have thus had a limited application in terms of biological insights or clinical applications. Second, complex, multiscale models are characterized by a reductionist point-of-view whereby general phenomena could be explained by decomposing them into elementary pieces. However, corresponding elementary experiments would not be suitable for quantification of the several homeostatic mechanisms involved in the whole real process. Thereby, we do not adhere to this reductionist vision and for modeling purposes we rather adopt a holistic approach considering the process as an indivisible whole.
To avoid the above-mentioned caveats, our methodology always starts from: 1) a clinically relevant medical problem but more importantly 2) the data available to build models.
In several instances, the mechanistic models are ordinary differential equations (ODEs). This is the case for the simplest type of experimental data that we generate, i.e., tumor growth kinetics. Departing from previous works establishing models for untreated experimental growth, we are now actively engaged into designing PK/PD models of the effect of multiple therapies. These models have to account the specificities of the drug delivery (e.g., nanoparticles), the biological effect of the treatments (e.g., cytotoxics, antiangiogenics or immunotherapies) and resistance to the therapy (either innate or acquired). The resulting models are novel nonlinear ODEs that need to be validated against the data and, when necessary, theoretically studied for their qualitative behavior. With the advent of immunotherapies, there has been a regain of interest to modeling tumor-immune interactions. Again, despite a wide literature on the subject, very few models have been validated against empirical data. A methodological objective is to establish and validate such models, including effect of immunotherapies.
Description of other phenomena are more adapted to partial differential equations (PDE) models. For instance, following an approach initiated by Iwata et al. 115, structured PDE models can be written for description of a population of metastases (see 4.3.2). Indeed, at the organism scale, cancer diseases are often characterized by a generalized (metastatic) state. However, few modeling efforts are currently focused on this aspect. The only validated models in large cohorts for systemic disease concern the sum of largest diameters as defined by the RECIST criteria 90. We aim to go beyond this state of the art by: 1) providing models of coupled tumor growth with interactions (and quantification of inter-lesion variability) 87, 88 and more importantly 2) developing models accounting not only for growth of the tumors, but also dissemination (birth of new lesions).
To date, most of the available and collectable clinical data about tumor growth and response to therapy consist of scalar data, often even limited to lesion diameters or sum of diameters. This is why we primarily focus our efforts on developing kinetic models of such data, the novelty coming from integrating other longitudinal biological data (e.g., from blood counts). Nevertheless, imaging data are now increasingly accessible and recent advances in image analysis allow the automatic segmentation of lesions make it possible to quantify the spatial shape and texture of tumors without a prohibitive cost for radiologists. This opens the way to develop spatially distributed PDE models of tumor dynamics. The existing models have largely remained unconfronted to data, apart from notable exceptions from the Inria MONC 97 and EPIONE 95 teams, as well as the Swanson 83 and Yankeelov 111 groups. Radiomics approaches quantifying heterogeneity in the images could bring additional information. We will rely on existing or establish collaborations with other dedicated Inria teams (EPIONE, MONC) for such purpose. COMPO would ideally bridge the gap between clinical studies and the Inria ecosystem. Finally, PDE models are also well adapted to describe intra-tumor drug penetration and we have recently developed such models for description of intra-tumor fluid flow and transport of antibody nano-conjugates 133.
3.3.2 Statistical modeling
Statistical models are defined here as mathematical constructs that describe the stochastic sources of variability in the data. They comprise both: 1) classical statistical models defining the functional and probabilistic relationship explicitly and 2) machine learning (ML) algorithms highly based on the data alone (e.g., tree-based models and associated ensemble methods or support vector machines) 108. We use such models for the following purposes: defining appropriate frameworks for parameter estimation; quantitative testing of biological hypothesis; addressing interindividual variability (using nonlinear mixed-effects (NLME) modeling); and building predictive models.
NLME – also termed the population approach in PK/PD modeling, or hierarchical modeling 117 – consists in assuming a statistical distribution of the parameters of the structural (often mechanistic) model, in order to describe longitudinal observations within a population of individuals. Instead of estimating individual parameters on a subject per subject basis – leading to identifiability issues in sparse data situations characteristic of longitudinal measurements in oncology – all data can be pooled together and a joint likelihood is obtained. Likelihood maximization becomes more complex than for classical nonlinear regression, nevertheless this problem has already been addressed by means of algorithms such as the deterministic first-order conditional expansion (FOCE) algorithm 118 or the stochastic approximation of the expectation-maximization algorithm (SAEM) 99. These algorithms are implemented in widely used software in the PMX community such as NONMEM® (Icon) or Monolix® (Lixoft), or R packages (e.g., saemix or nlme). Once the population distribution is estimated, empirical Bayes estimates (EBEs) can be derived for estimation of individual parameters. We also use the language Stan that implements state-of-the art Bayesian methods 91.
Departing from a general distribution of the parameters (often assumed log-normal) with quantified but unexplained interindividual variability, covariates are incorporated to explain this variability and build predictive models. This is traditionally done by means of linear models (possibly up to a functional transformation). However, with the increase in number of such covariates, the traditional tools and algorithms are limited. We thus develop advanced covariate models in NLME incorporating ML algorithms. Such methods require novel contributions. A possible lead is to first identify the EBEs and then use ML algorithms to predict these from the covariates 125. In other cases, ensemble models could be built from the heterogeneous sources of data, integrating one sub-model from EBEs identified from early data. Another, more challenging avenue would be to adapt the parameter estimation algorithms like SAEM to include ML models in the covariate part.
In addition, because few data have been available longitudinally so far (i.e., small number of quantities measured at each time point), the current use of NLME relies on models with a small number of output variables. In this respect, modern clinical oncology studies bring new modeling and statistical challenges because many more quantitative data are collected at each time point (e.g., hundreds of variables from immuno-monitoring or possibly tens of thousands from circulating DNA, or radiomics features from imaging). Defining high-dimensional ODE models describing all the physiologically meaningful variables becomes intractable, therefore new methods are required. A possible avenue is to have a sequential approach, using first ML methods to reduce the dimension, then model the reduced number of variables. Another, more challenging, avenue would be to perform the two tasks (dimension reduction and temporal modeling) at the same time, and include this in an NLME framework for population estimation. The first part could be done using tools from unsupervised learning such as auto-encoders.
Following the availability of longitudinal tumor measurements, recent developments in the field of NLME have concerned joint modeling 101. This consists in modeling the longitudinal kinetics of a biomarker (e.g., tumor size) together with censored time-to-event data (e.g., overall survival) in a single step. Promising results have been obtained so far and we intend to develop methods beyond the state-of-the art in this area. This includes, in connection with above: 1) extension to models with emergence of new metastases, 2) integration of high-dimensional covariates and 3) high-dimensional longitudinal data. This Bayesian integration of data for updated survival predictions could lead to high impact results, as demonstrated by a recent publication in Cell 116.
Finally, we intend to bring the use of established ML tools to address concrete clinical problems emerging from the data collected in routine or clinical trials. Indeed, such data is so far analyzed using traditional statistical methods. ML algorithms could bring added value for predicting efficacy or toxicity from demographic, clinical and biological data.
3.4 Experimental therapeutics in oncology
The project-team is based upon generating experimental and clinical data to identify and test the models, and to provide proof-of-concept studies so as to validate the model-based dosing and scheduling prior to transposing them in patients. Historically, experimental therapeutics in oncology has relied on a wide variety of in vitro and in vivo models mimicking human cancer disease. In oncology, hundreds of in vitro models using cancer cell lines cultivated following 2D or 3D (spheroids) fashion, plus more sophisticated models with cancer cells enriched with fibroblasts or endothelial cells 126, eventually leading to complex organoids 105. Similarly, almost all kind of tumors can be tested in vivo, mostly in small rodents. In oncology, in vivo models are mostly based upon xenografting human tumors from established cell lines or from patient biopsies (patient-derived xenografts or PDX) so as to better mimic human pharmacology when testing active compounds next. To achieve this, several strains of immune-compromised mice have been successfully developed. Because immune checkpoint inhibitors do not exert direct anti-proliferative activity on cancer cells but are rather expected to harness tumor immunity, human xenografts in immuno-compromised mice is not anymore a suitable model. This has led investigators to shift towards immune-competent syngeneic mice models. Non-clinical experiments with drug candidates in immunotherapy mostly focus on deciphering the pharmacology of the targeted pathways, assessing the cytokine release potential, studying receptor occupancy, by using models the most likely to mimic tumor immunity in human. More sophisticated animal models such as human knock-in mice, immuno-avatar, hemato-lymphoid humanized mice or immune-PDX mice have been developed 129 (i.e., allowing to test immune checkpoint inhibitors in mice models combining human xenograft with relevant, humanized immunity and stroma cells) have been made available as well. Beyond generating data on efficacy such as reduction in primary tumor mass or metastatic spreading, experimental models help providing as well in depth knowledge on human and animal target cells, in vitro and in vivo concentration-effect studies, search for biomarkers, plus the most comprehensive knowledge on animal vs. human differences on dose – exposure – effects relationships and finally drug distribution throughout the body, target expression, affinity of target-binding and intrinsic efficacy, duration and reversibility of the effects. In particular, animal drug metabolism and pharmacokinetics (i.e., exploration of liver metabolism and distribution / absorption processes using in vitro or in vivo dedicated models) help understanding the disposition and distribution of the drug in the body throughout time, especially its ability to target tumor tissues (i.e., in vivo distribution in tumor-bearing mice) and helps understanding sources of pharmacokinetic variability. All this information requires state-of-the-art techniques for measuring drugs and drug metabolites into biological fluids in tissues, such as fluorescence-imaging, high-performance liquid chromatography or liquid-chromatography-mass spectrometry bioanalysis. Our team has proven track records in the field of experimental therapeutics in oncology, with two PhDs on developing anticancer nanoparticles in breast and colorectal cancer 104, 128 plus experiments on model-driven way to combine anti-angiogenics with cytotoxics in breast and lung cancers 114, 121, 130, model-driven determination of alternate dosing in neuroblastoma, or methodological studies on monitoring tumor growth 122.
3.5 Axis 1: Quantitative modeling for personalized clinical oncology
The different steps of patient therapeutic management by clinicians consist mostly of: diagnosis, estimation of the extension of the disease, choice of therapy and evaluation of the therapy (efficacy, toxicity). This axis is specifically concerned with such clinical problems, apart from the pharmacological aspects addressed in the other axes.
In this axis, we aim to develop mathematical and statistical models and methods able to process this information to bring added value by inferring hidden parameters and provide simulations and predictions about the past and future behavior of the disease.
In the short-term (4 years), our research projects are: (1) modeling large-scale longitudinal data from immuno-oncology for prediction of response to immune checkpoint inhibition (QUANTIC and TGI-ML projects), (2) developing clinically relevant mathematical models of metastasis and (3) modeling the kinetics of clinical biomarkers.
3.6 Axis 2: Individualizing anticancer drugs dosing regimen
This axis fits within the “population approach” introduced in the 80’s by L. Sheiner 121 and aims at gathering exhaustive information about the multiple sources for variability in response in patients (including but not limited to drug-drug interactions, pharmacogenetics, and cormorbidities affecting renal and liver functions), build specific mechanistic models including relevant covariates and determine the PK/PD relationships of drugs used in oncology-hematology or for treating solid tumors. This covers cytotoxics, oral targeted therapies, biologics or immune checkpoint inhibitors. The overall goal is to achieve precision and personalized drug administration, i.e., the right dosing and scheduling regimen for the right patient.
In the short-term (4 years), our research projects will be focused on the following objectives: 1) predict response or toxicity variability dependent on pharmacogenetic (PGx) and pharmacogenomic data, 2) assess PMX of anticancer agents such as biologics, including ICI, and 3) develop physiologically-based PK models of nanoparticles distribution. Together, these objectives will allow to gain insights in the variability in drug response that depends on PK (1, 2 and 3) or germinal genetic alterations (1).
3.7 Axis 3: Optimizing combinatorial strategies with immune checkpoint inhibitors
Our hypothesis is that so many attempts to combine drugs fail not because the underlying pharmacological concepts are wrong (such as immunogenic cell death triggered by cytotoxics or radiation therapy, or increase in T cells infiltration with anti-angiogenics) but because these combinations probably require fine tuning in terms of dosing, scheduling and sequencing, whereas in practice all the drugs are given the same day. The goal of this second axis is therefore to shift from current empirical and suboptimal combinatorial regimen to model-informed designs to best combine drugs and therapeutic approaches so as to maximize efficacy while controlling toxicities. To do so, we will rely on our pioneering work about model-driven scheduling in early phase trials for combination of cytotoxic agents in metastatic breast cancer (MODEL1 trial) 109, 120 and metronomic vinorelbine in lung cancers (MetroVino trial) 86, 103. Leveraging the unique multidisciplinary aspect of our team, we implement a fully translational approach going from experimental therapeutics, PMX and quantitative systems pharmacology, to clinical trials either in early (phase I/II) or late (phase III) settings. Of note, our group has already an expertise in developing mathematical models determining the best sequencing between chemotherapy and anti-angiogenics.
In the short-term (4 years), our research projects will be focused on providing model-informed designs for combining ICI with: (1) cytotoxics, (2) an experimental immunoliposome and (3) radiotherapy.
3.8 Mid-term objectives
3.8.1 Modeling
We plan to achieve two main things on the modeling side: 1) the development of effective numerical tools (either as web applications or as part of simulation software) and 2) the empirical validation of the models.
For 1), this includes a tool able to predict response to ICI monotherapy in NSCLC from baseline and early response data . We will work with our industrial partners (in particular, HalioDX from the PIONeeR consortium), to transfer the tool for commercial use. Second, We plan to have a validated numerical tool able to predict metastatic relapse from clinical biomarkers at diagnosis, using our mechanistic model. This model will integrate the effect of adjuvant therapy (hormonotherapy or cytotoxic therapy) and will be able to simulate the long-term impact of alternative treatments (e.g., number of cycles to be administered to prevent distant relapse). It will have been validated from our local databases and will be implemented in a clinically-usable online tool such as PREDICT 134. The main difference with this tool will be the ability to mechanistically simulate the effect of therapy. We plan to have initiated a larger initiative at the national or European level to collect large data bases, validate further the predictive power of the model, and refine its structure if required. We will also extend this tool to other pathologies that share the same problematic (diagnosis at early-stage, important probability of future distant relapse) such as kidney cancer, following our initial work from preclinical data 84, 89, 98.
The pharmacometric models that will have been developed will also be implemented as clinically effective dose adaptation numerical tools directly usable to personalize the dose and scheduling of multiple anti-cancer agents not only by the clinicians and pharmacists from our group, but also by others, at least at a regional level.
For 2), our strategy of validation is the following. First, during the development phase, a proportion of the dataset (usually, 30%) is left aside unused for establishment of the model and initial calibration, and then used as a test set. When the sample size is too small (n 100 patients), only (nested) cross-validation is employed to assess the predictive power of the model. Evaluation metrics will be the classical ones, adapted to the task (classification, regression or survival regression) and include discrimination and calibration. In addition, specific methods will be used when in the context of mixed-effects approach (e.g., visual predictive checks for evaluation at the population level). The second step consists in evaluating the predictive power of the models in retrospective, external data sets. We have for instance initiated a collaboration with Dr C. Scherer (Clermont-Ferrand) to validate our metastatic prediction model on an external database of 3061 patients. The third step is to validate the added value of the model-based approach compared with the standard of care and is a long-term rather than mid-term objective.
3.8.2 Pharmacological and clinical oncology
In axis 2, in addition to standard drugs, developing tools for similarly better understanding the sources of therapeutic and PK variability and understanding the PK/PD relationships of cell therapy in oncology such as CAR-T cell therapies, is a challenging task. The challenge with CAR-T cells is that first, developing bioanalytical tools to monitor them in patients is not trivial, and second, little but nothing is known regarding their PK properties and possible sources impacting on PK/PD relationships such as disease status or immune status of the patient. We aim at developing both a platform to monitor CAR-T cells and future cell therapies in plasma and mechanistic models to describe the disposition of these new therapies in the body.
The nanoPBPK model will be extrapolated to humans and used to determine the specifications of an optimal nanosystem in order to penetrate solid tumors such as pancreatic tumors. The rationally designed nanosystem will be evaluated in vitro and in vivo in order to validate the approach. The nanoPBPK model will be interfaced to become a software and be shared with the scientific community. To achieve this goal, a partnership with ESQlabs, the company developing the opensource PBPK platform PKSim, has already been approved by both sides. The nanoPBPK model will be combined with pharmacodynamic modelling describing the effect of the loaded anticancer drug on tumor growth and metastases spread, on the immune system, and on dose-limiting toxicities.
Our mid-term objective in axis 3 is to assist the design of scheduling regimen for combinatorial treatments in early phase clinical trials, which represent an important clinical challenge of the next 10 years. To do so, we will benefit from our close connection to the INCa-labeled AP-HM's center for early phase clinical trials (CLIP2). Our aim is to design model-based, individualized and adaptive scheduling regimen that depend on the monitoring of the disease evolution. We plan to run phase I/II trials based on the model recommendations. Depending on our achievements and success in phase I/II trials our mid-term goal would be to lead a prospective, randomized, phase III trial comparing a model-based adaptive regimen to the standard of care for combination of immune checkpoint inhibition with chemotherapy and/or anti-angiogenic and targeted therapy. According to our team expertise, the target malignancies would be primarily lung cancer (LG) and head and neck cancer (SS).
3.9 Long-term objectives
At long-term, we globally wish to have established a worldwide leader position in the fields of quantitative mathematical oncology and PMX, as well as the pharmacokinetics of nanoparticles. We hope that this would translate into the achievement of three goals: (1) the development of software effectively used for clinical decision-making and dosing adjustment (estimated achievable), (2) the initiation of prospective, phase III clinical trials comparing model-guided therapy versus standard of care (highly challenging), (3) clinical trials of nanosystems designed by our group (estimated achievable). In addition, we foresee several avenues both in terms of modeling opportunities and applications.
3.9.1 Modeling
Our short-term program is devoted to the development of new models and their confrontation to empirical data. The mid-term program will focus on the validation and refinement of these models. In the long-term, we foresee that this will bring novel questions in terms of mathematical analysis of the models. For instance, metastatic modeling (4.3.2) will establish validated models for tumor-tumor interactions, including immune-mediated interactions (4.3.1). In turn, this leads to nonlinear, size-structured, renewal PDEs. Study of the asymptotic behavior of such equations is non-trivial.
More generally, we expect that physiologically structured PDEs (psPDE) can become relevant to practical modeling in oncology, from two types of data: flow cytometry and single-cell sequencing. Flow cytometry is currently becoming of increasing relevance to characterize multiple populations of cells, for instance in the context of immuno-oncology. In the QUANTIC project (4.3.1), we are starting to interact with such data, only by means of scalar quantities so far. However, the structure of this data is to have, for each cell, a quantitative measure (e.g., a surface marker). Measuring these in a population of millions or billions of cells makes it adapted to modeling by such psPDE. Similarly, single-cell sequencing is a technique by which every cell of a population (e.g., in a tumor) is sequenced, thus having mutation information. In turn, this allows to quantify subclones in the population. Such data has already generated fascinating results, for instance in the study of metastatic development theories 107, 113. Although evolutionary modeling is a wide field with established groups (M. Nowak or F. Michor in Harvard, C. Curtis in Stanford, T. Graham at the CRUK), few groups are modeling dynamical data at single-cell resolution. To this regard, the theoretical work initiated by J. Clairambault and B. Perthame (Inria MAMBA) suggesting to use psPDEs to model evolution in cancer cell populations could be appropriate 92. Parameter estimation in such models is a challenging task 102 and data assimilation from flow cytometry or single-cell sequencing data sets would represent an important avenue. Dynamical data can be provided by circulating tumor DNA and we have already initiated contacts with an important clinical and biological study in Marseille on this topic (the SCHISM study, PIs: SS and F. Fina). The recent developments of technology enabling spatial resolution of single-cell sequencing also paves the way to exciting avenues in terms of modeling 123.
We will also build models that can optimize the effectiveness of treatments incorporating new criteria (other than the evolution of tumor mass) of diagnostic and therapeutic evaluation, especially those we have forged around the information provided by functional imaging (T80 computational algorithm time at which 80% of FDG is metabolized 85, 96, 127.
3.9.2 Pharmacological and clinical oncology
A general, challenging, long-term objective, would be to run prospective clinical trials in which a model-informed arm would be compared to the standard of care. In the model-informed arm, therapeutic decision would be based on the recommendation of the model. This applies to the models developed in all axes. For instance, in breast cancer, the number of cycles of chemotherapy would be adapted based on the model indication (axis 1), decision of the maximum tolerated dose in the treatment of leukemia patients would be based on the PGx/PK/PD model (axis 2) or the combination scheduling regimen would be given by model calculations (axis 3).
Regarding nanosystems initially designed by our group based on the nanoPBPK modelling, they will also be tested in early clinical trials. Our group will drive the design of these based on simulations performed with the nanoPBPK and pharmacodynamic model, in order to guarantee the highest chances of success while ensuring patients safety. In particular, nanoparticles specifically transporting cytoxics could replace standard systemic myelo-ablation in hematopoietic stem cell transplantation, a risky strategy with frequent life-threatening, when not lethal, toxicities. Because of the fully controlled distribution phase in the body, nanoparticles encapsulating several drugs could thus be implemented in the preparative regimens for allogeneic stem cell transplantation in leukemia or myeloid malignancies.
In addition, several groups predict that in addition to standard drugs or biologics, or rising gene therapy and cell therapy strategies, new devices such as nanobots will be developed to treat cancers. Nanobots are entities which are not designed to interact with standard pharmacological targets or genes like current anticancer treatments, but could fix the cancer cell, either by providing a missing protein, or ultimately trigger a mechanic cell-death using radiation, thermal wave, or by disrupting cell membrane. These new entities should exhibit totally new pharmacokinetics, because they are unlikely to be metabolized in the liver or to be cleared by the kidney or the biliary tract. Therefore, new models for PK/PD should be developed, because neither behavior in the body or intrinsic mechanisms of action are known yet. In addition, the issue of nanosafety with such devices will be particularly critical and will require extensive PMX resources, to predict long-term effects or to keep under control the mechanism of action. Should such nanobots be developed, the COMPO joint-team should develop specific resources to monitor their fate in the body, specific resources to write equations describing nanobots/body, nanobots/immune system, and nanobots/cancer cells interactions, plus new global models encapsulating all the interactions, and pharmacodynamics impact of such devices.
Depending on the expertise we gain in the PK/PD knowledge of those cell therapies as part of the mid-term objectives, optimizing combinatorial strategies to such cell therapies will be another long-term objective.
Last, pharmaco-economic studies including impact of quality of life, increase in both tolerability and efficacy will be performed to determine whether PMX-based dosing is a cost-saving strategy. For instance, by refining the scheduling of immune checkpoint inhibitors, such as determining, using modeling and simulation strategies, when the plasma concentration of the drug reaches the threshold in trough levels necessary to ensure maximal target engagement, it will be possible to customize the frequency of the administrations, with possible strong impact on treatment costs. By using real-life data, we propose to use dedicated models to quickly define alternate and more appropriate dosing and/or scheduling with newly marketed anticancer agents, either chemotherapy, targeted therapies or biologics including immune checkpoint inhibitors.
4 Application domains
The COMPO research team's projects all focus on a serial of complementary and inter-related domains described in an itemized fashion below:
- Health: all the models to be developed within the framework of the COMPO team are related to improving healthcare;
- Cancer: in particular, the models will be developed to address specific issues related to cancerous diseases;
- Precision Medicine: in particular, in cancer the developed models will be part of the implementation of precision medicine in oncology focusing on the following items;
- Combinatorial regimen: developing model-informed strategies to determine the optimal modalities when combining several treatments altogether. With the increasingly diversified arsenal of therapeutic approaches to treat cancers (surgery, radiotherapy, chemotherapy, targeted and anti-angiogenic therapy and immunotherapy), defining optimal combination protocols is highly challenging 93. This spans the issues of sequencing, scheduling and dosing of those therapies, which are to date largely addressed using a trial-and-error approaches. Consequently, too many combinatorial trials fail, and attrition rate with combinatorial immunotherapy is now a rising issue in clinical oncology and we hypothesize that extensive modeling and pharmacometrics could help refining the way anticancer drugs are combined;
- Tools for decision-making: developing model-informed strategies to forecast clinical outcome, i.e., during clinical trials. Assessing the predictive power of markers not only at baseline but also in their change over time is a current challenge. The information available, on the basis of which decision is made, includes clinico-demographic variables, classical biomarkers (e.g., blood counts, thyroglobulin, lactate dehydrogenase levels, etc...) but also an increasing amount of data from other sources (e.g., immuno-monitoring, anatomical functional imaging or genomics) that require state-of-the-art modeling to analyze extremely dense and longitudinal data;
- Adaptive dosing strategies: developing model-informed strategies to customize dosing so as to ensure an optimal toxicity-efficacy ratio. All anticancer agents are approved upon registration trials performed in highly selected patients (i.e., with controlled lifestyle, little comorbidities, controlled polymedication and restricted range of age), thus smoothing the interindividual variability. In real-world practice however, patients are all heavily co-treated with a wide variety of other drugs plus herbal medications, likely to interact with drug metabolism and transport, and are frequently older than in clinical trials. In addition, genetic polymorphisms affecting genes coding for drug transport proteins or drug-metabolizing enzymes in the liver, or transcriptional factors can impact as well on dose-exposure relationships. Consequently, standard dosing may not be suitable in non-standard patients to reach the adequate drug exposure levels associated with optimal toxicity/efficacy balance;
- Nanomedicines: developing model-informed strategies to conceptualize drug-loaded nanoparticles likely to improve the toxicity-efficacy ratio over conventional treatments. As of today, the biodistribution phase of anticancer agents is totally aspecific, making "on-taget off-site" actions an issue because it is associated with drug-related side effects affecting healthy tissues. Nanoparticles present unique features likely to deliver specifically a high amount of payload directly on a tumor site, thus improving the antiproliferative action while sparing healthy tissues. In addition, nanoparticles are expected to reshape the tumor micro-environment, making them good candidates to be further associated with immunotherapy (see Combinatorial Regimen above).
5 Highlights of the year
- Communication of results from two large scale studies using mechanistic modeling and statistical learning for prediction of outcome (response and/or) survival following immunotherapy (immune-checkpoint inhibitors, ICI) in non-small cell lung cancer.
- Prediction of survival following ICI using a train set of three phase 2 trials (862 patients) and validated on a phase 3 trial (test set, 553 patients), using baseline(RNAseq + clinical + mutation) and longitudinal (tumor and biological markers kinetics) 48, 49, 79, 78.
- Multi-modal (tissue: multiplex immunohistochemistry + blood: immune-monitoring, hematology, biochemistry, vasculo-monitoring + routine clinical variables) predictive signatures of response to ICI in either the second+-line 55 or first-line 46 setting.
- Communication of results from a Phase I/II clinical trial entirely driven by upfront mathematical modeling
- Metronomic Vinorelbine was succesfully administered using a model-driven regimen in heavily pre-treated lung cancer patients with 73 percent Disease Control Rate. NLR ratio as a predictive marker for efficacy suggests underlying immunomodulating features of metronomic regimen. 12.
6 New software and platforms
6.1 New software
6.1.1 nlml_onco
-
Name:
Nonlinear mixed-effects modeling and machine learning for oncology
-
Keywords:
Nonlinear mixed effects models, Machine learning, Oncology, Survival analysis
-
Scientific Description:
- Exploratory data analysis
- Automated mixed-effects modeling analysis
- Preprocess
- Analysis of high dimensional transcriptomic data
- Dimension reduction
- Feature selection
- Survival machine learning algorithms
- Applications (> 3,000 patients)
- Individual predictions of survival
- Predictions of hazard ratios for applications in drug development, for both monotherapy and combinatorial treatments
-
Functional Description:
This software analyses multiple data arising from clinical oncology (routine care and clinical trials). This data can be of two types: 1) static (e.g., baseline features), from clinical, biological, molecular (e.g., transcriptomic or mutation data) 2) longitudinal (multiple time points per individual): tumor kinetics, biomarkers The second type is modeled using the framework of nonlinear mixed-effects modeling. All features are then analyzed using data science techniques (preprocess, feature selection, machine learning algorithms), in order to predict survival outcome.
-
Release Contributions:
Established the first clean version of the software with multiple use cases.
Checked that all runs and produces the expected outputs.
Separated code from input (data) and output files
-
News of the Year:
Released clean and documented version to the industrial partner. Multiple use cases documented spanning the full range of use.
Multiple improvements for results post-processing in order to generate: 1) the final report of the collaboration project, 2) oral communications to major conferences and 3) main publication.
- URL:
- Publications:
-
Contact:
Sebastien Benzekry
-
Participant:
Sebastien Benzekry
-
Partner:
Roche
6.1.2 ml.tidy
-
Name:
Machine learning with tidymodels
-
Keywords:
Survival analysis, Machine learning, Data analysis, Oncology
-
Scientific Description:
This software maximises the use of the R package tidymodels.
-
Functional Description:
This software analyses data generated by several partners in the context of the PIONeeR clinical study. It can be either used to perform feature selection, plot feature importances, run machine learning algorithms mostly for survival analyses, post-process these predictions or perform learning curves.
-
News of the Year:
First machine-learning analyses of the PIONeeR data. Establishment of biomarkers signatures predictive of the early progression and/or of the progression-free survival, for different cohorts of the same clinical study. Cross-validated and potentially further validated these signatures.
- Publication:
-
Authors:
Sebastien Benzekry, Melanie Karlsen
-
Contact:
Sebastien Benzekry
6.1.3 stats_pioneer
-
Name:
Statistical analysis and reporting of the PIONeeR study
-
Keywords:
PIONeeR, Statistical analysis, Biostatistics
-
Functional Description:
This software was built to analyse the PIONeeR (Precision Immuno-Oncology for advanced Non-small cell lung cancer patients with PD-(L) 1 ICI Resistance) data. PIONeeR is a prospective, multicenter study with primary objective being to validate the existence of a hypothetical immune profile explaining resistance to immunotherapy in non-small cell lung cancer patients.
This software corresponds to the very first step of the data analysis, which is the statistical analysis. Some of its functions aim at: - preprocessing the data (creation of clinical variables, dictionary, outcome variables, clinical biomarkers, treatment of the variables types) - computing statistical tests, logistic or Cox regression, or performing a correlation analysis - visualising the data (boxplots, barplots, survival curves, ROC curves, volcano plots) - providing detailed and interactive statistical reports on the data - automating the production of these reports using Gitlab CI/CD
-
News of the Year:
# 2022
## Software
- Receipt of a new batch of patient data (data lock 3)
- Cleaning of data
- Integration of these patients in the analysis
- Visualisation of data
- Preliminary study of missing data
- Interactive tool for the study of correlations between variables
- Refinement of the statistical analysis techniques used
- Automatic generation of statistical analysis reports
- Production of reports
- Use of Docker containers for reproducibility
- Integration with Gitlab CI
- Deployment of reports
- Integration with Gitlab Pages
- Provision of restricted website access to partners via Gitlab guest accounts
## Communications
- Poster communication in AACR 2022 (second+-line patients)
- Oral communication to ESMO-IO (first-line patients)
- Publications:
-
Authors:
Sebastien Benzekry, Melanie Karlsen, Kokou Atsou, Paul Dufosse
-
Contact:
Sebastien Benzekry
6.1.4 metastats
-
Keywords:
Nonlinear mixed effects models, Mechanistic modeling, Metastasis, Cancer, Survival analysis, Data analysis
-
Functional Description:
Code to fit a mechanistic model of time to metastatic relapse to clinical data, using mixed-effects statistical learning. It contains simulation of the natural history of primary and secondary tumors. Statistical population distributions of the parameters can be fitted to describe right censored time-to-event data. Covariates can be included to perform predictive modeling. Evaluation of predictive performances (c-index, calibration plots and classification metrics) are implemented.Cox and survival random forest analysis are also implemented.
-
Release Contributions:
New features: Tools for identifiability Fixes: A lot of documentation added and/or fixed Removed python implementation Plot fixes
-
News of the Year:
2022: Tools for identifiability
2020/2021: The code was totally reworked and bundled as an R package to run and fit the model. The simulation code was ported from a python prototype to an optimized c++ version directly available from R. This new version of the model code enabled considerable gains of computational time when fitting the mixed effect model. A particular attention was ported on the package architecture to make it as easy as possible to use and maintain. The model was used on breast cancer data from several databases (Bergonié Institute, AP-HM, open-access data) to assess its performances. The results will be the subject of a communication in the 2022 AACR annual connference and of a later publication.
- URL:
- Publication:
-
Contact:
Sebastien Benzekry
-
Participants:
Celestin Bigarre, Chiara Nicolo, Sebastien Benzekry
-
Partner:
Institut Bergonié
6.1.5 metamats_size
-
Name:
Fitting longitudinal data of size and number of metastases using mechanistic models
-
Keywords:
Cancer, Mechanistic modeling, Metastasis, Regression, Simulation, Data assimilation
-
Scientific Description:
This software fits models of metastatic development to longitudinal data of metastatic sizes and provides simulation and visualization tools for metastatic modeling.
-
Functional Description:
This software fits models of metastatic development to longitudinal data of metastatic sizes and provides simulation and visualization tools for metastatic modeling.
-
News of the Year:
2022: Port of the code to R Application of the model on a new dataset of patients (50 patients with SCLC, brain metastases)
Previous years: Development of the software to analyze a database of 30+ patients. Addition of features to predict overall and prediction free survival from the mathematical parameters
- URL:
- Publication:
-
Contact:
Sebastien Benzekry
-
Participants:
Sebastien Benzekry, Mariia Bilous, Celestin Bigarre
-
Partners:
Centre de Recherches en Cancérologie de Marseille, Institut Bergonié, Assistance Publique - Hôpitaux de Marseille
6.1.6 q_single_cell_tools
-
Name:
qPCR single cell analysis tools
-
Keywords:
Single cell, Data analysis, Bioinformatics, Data visualization
-
Functional Description:
qSingCTools is a web application which allows the pre-processing, analysis and visualization of qPCR Single Cell data. qSingCTools takes a Gene X Cell table of CT values generated by qPCR experiments. Gene expression values were then calculated by applying the y=40-CT formulate. The count values equal to 999 (or missing values) were substituted by values generated from a Normal distribution centered on zero with a standard deviation obtained from the dataset.
-
News of the Year:
The current version of qSingCTools can manage multiple datasets (until 3 tables) which are merged together before analysis. qSingCTools allows normalization between datasets based on geNorm algorithm (Vandesompele et al., Genome Biology, 2002) using the housekeeping genes (HKG) present in the dataset.
- URL:
-
Contact:
Abdessamad El Kaoutari
6.1.7 pacaomics explorer
-
Name:
PacaOmics data explorer
-
Keywords:
Transcriptomics, Cancer, Data visualization, Bioinformatics
-
Functional Description:
The app allows the comparison of the gene expression level vs the PAMG which is a transcriptomic signature that describes PDAC heterogeneity as a continuous gradient from pure basal-like (low PAMG) to pure classical phenotypes (high PAMG).
-
News of the Year:
The 1st version of the this shiny application allow user-friendly exploration and visualisation of expression data (RNAseq) generated from pancreatic adenocarcinoma (PDAC) patients using pre-clinical models including PDX, primary cell cultures and organoids.
-
Contact:
Abdessamad El Kaoutari
7 New results
7.1 Mechanistic learning for prediction of outcome after therapy
7.1.1 Supporting decision making and early prediction of survival for oncology drug development using a pharmacometrics-machine learning based model
Participants: Sébastien Benzekry, Mélanie Karlsen, Abdessamad El Kaoutari, Rene Bruno, Ales Neubert, François Mercier, Martin Stern, Bruno Gomes, Suresh Vatakuti, Peter Curle, Candice Jamois.
Funding and data: Roche pRED
BACKGROUND: Despite some recent advances in cancer treatment, the attrition rate in late phase studies remains high. Predicting response to therapy using readouts from early studies where data are often limited and patients' diseases are very advanced remains a challenge. However, host fitness and tumor biology biomarkers could be good prognostic indicators of survival. Early on-treatment changes could improve survival prediction compared to baseline factors only. Nonlinear mixed effect (NLME) models are useful to leverage longitudinal data and can help identify trends in observed data. Nevertheless, these models can be limited for data mining purposes and machine learning (ML) could help.
OBJECTIVES: (1) Integration of a large database composed of baseline patients’ and disease characteristics, longitudinal lab parameters and tumor size data, genomic and transcriptomic data from patients (pts) with advanced non-small cell lung cancer treated with atezolizumab (ATZ); (2) Development of a NLME-ML based model for early prediction of survival; (3) Study risk prediction
METHODS: The model was trained on data from 3 ATZ phase 2 trials (862 pts), and validated on OAK phase 3 trial (553 pts).
Model development: Training data consisted of baseline clinical variables (p=73), transcriptomic and mutational data (p=58,311 transcripts and 395 genes), longitudinal data for tumor size (TK, 5,570 data points) and four pharmacodynamic (PD) markers: neutrophils (ANC), C-reactive protein (CRP), lactate dehydrogenase (LDH) and albumin (61,296 data points). NLME models were used to describe TK and PD parameters time courses. Individual empirical bayes estimates (EBEs) from each model parameters, clinical factors, transcriptomic and mutational data were used as features for the survival ML models. Dimensionality reduction and features selection methods were applied to identify a “minimal signature model”. Several algorithms of survival prediction and classification (survival at 12-Months landmark) were tested using nested cross-validation [11-12]. Various performance metrics were evaluated (e.g., C-index, area under the curve (AUC), sensitivity (SE), specificity (SP), positive and negative predictive values (PPV, NPV), survival and calibration curves).
Model validation: The model was applied to pts' data from the ATZ (N=396) and docetaxel (DTX) (N=354) control arm in OAK. Individual EBEs from each model parameters identified using Bayesian estimation were used as features. Data were truncated using the time from randomization of the first patient (5 - 100 weeks) to mimic real on-study conditions. Predicted survival hazard ratio (HR) was compared to observed survival: for each patient and both arms (ATZ and DTX), the ML model was used to generate 100 replicates of predicted survival curves for each pt and subsequently 100 study-level survival curves. Mean (95% prediction interval (PI)) was compared to observed HR (and 95% confidence interval (CI)).
RESULTS: The best NLME and ML models were double-exponential (TK, ANC, CRP, LDH), hyperbolic (albumin) models and Random Survival Forest (RSF) algorithm. The minimal signature model was composed of 26 features: 11 routine clinical variables, 3 TK and 12 PD model derived parameters. It exhibits good predictive power (C-index = 0.818 ± 0.029, AUC = 0.905 ± 0.0414) that was significantly improved when PD model metrics were added to baseline variables or TK parameters. RNAseq data had low predictive power. Model simulations were able to reproduce retrospectively the survival curves of ATZ and DTX arms from OAK, despite the different mode of action of each drug (anti PD-L1 checkpoint inhibitor and taxoid antineoplastic agent) and accurately predicted the study outcome with a predicted HR (95% PI) of 0.765 (0.692 - 0.829) versus observed HR (95% CI) of 0.765 (0.64 - 0.913). Applied to partial data, the model was able to predict ATZ survival benefit over DTX already at 8 months (30 weeks) post randomization and predicted HR (95% PI): 0.802 (0.655 - 0.907) versus observed HR (95% CI): 1.04 (0.386 - 2.79). ATZ’s survival benefit over DTX was seen in the data from 21 months onwards.
CONCLUSION: This NLME-ML based model could inform the decision to move an asset to a later phase of development through the analysis of early data. Its ability to predict survival in both treatment arms from OAK holds the premise for a potential extrapolation to other drugs within the same disease setting.
7.1.2 Comprehensive biomarkers (BMs) analysis to predict efficacy of PD1/L1 immune checkpoint inhibitors (ICIs) in combination with chemotherapy: a subgroup analysis of the Precision Immuno-Oncology for advanced Non-Small CEll Lung CancER (PIONeeR) trial
Participants: Fabrice Barlési, Laurent Greillier, Florence Monville, Clarisse Audigier Valette, Stéphanie Martinez, N. Cloarec, Sylvie van Hulst, L. Odier, Frédéric Vely, L. Juquel, Laurent Arnaud, S. Bokobza, Mourad Hamimed, Mélanie Karlsen, Paul Dufossé, Amélie Pouchin, Lamia Ghezali, Maryannick Le Ray, Jacques Fieschi, Sebastien Benzekry.
Funding : This work is supported by French National Research Agency (ANR-17-RHUS-0007, a partnership of AMU, APHM, AstraZeneca, Centre Léon Bérard, CNRS, Veracyte, ImCheck Therapeutics, Innate Pharma, Inserm, Institut Paoli Calmettes and sponsored by AP HM and initiated by Marseille Immunopole. It is also supported by Plan Cancer Inserm grant number 19CM148-00 as part of the QUANTIC project.
Publication: 46.
BACKGROUND: Prediction of ICIs efficacy in combination with chemotherapy remains an unmet need in patients (pts) with advanced NSCLC. The PIONeeR trial aims to predict response/resistance to PD1/L1 ICIs through a comprehensive multiparametric BMs analysis.
METHODS: We focused on the first 155 ECOG PS0-1 pts treated with pembrolizumab in combination with platinum-based chemotherapy as 1st line therapy. Tumor tissue was collected at baseline and pts were re-biopsied at 6 weeks, and blood-sampled every cycle throughout 24 weeks. Immune contexture was characterized in tumor & blood through FACS for circulating immune cell subtypes quantification and endothelial activation, blood soluble factors dosage, dual- & multiplex IHC / digital pathology to quantify immune cells infiltrating the tumor, WES for TMB & ICI plasma pharmacokinetics, leading to 298 assessed BMs. Multimodal data integration through supervised machine learning (SML) was performed with bootstrap LASSO on a train (N=116) and a test dataset (N=39) to establish a BMs signature able to predict progression-free-survival (PFS) at 1 year.
RESULTS: Pts were mainly male (65%), smokers (96%) and <70yrs (82%). Tumors were mainly nonsquamous (87%) with PD-L1 TPS>1% in 38.4% of cases. With a median follow of 11.4 months, median PFS was 9.8 months and median overall survival was not reached. Using baseline data, SML identified a 15 BMs signature including classical (age, ECOG PS, PD-L1 TPS…) but also experimental parameters (CD45+ CD16+ cells density in tumor, CD45- CD73+ cells density in stroma, tissue factor and CD31+ CD41+ AnC+ microparticles blood concentrations…) with high predictive performance for PFS. On the train dataset, C-index was 0.79 ± 0.13 and AUC was 0.81 ± 0.28. These scores were confirmed on the test dataset, with C-index of 0.80 and AUC of 0.84.
CONCLUSION: The PIONeeR trial provides a novel comprehensive BMs analysis to establish predictive models of response/resistance to ICI in advanced NSCLC pts. Combination of BMs can individually predict outcomes of chemo-immunotherapy.
7.1.3 Comprehensive biomarkers analysis to explain resistances to PD1/L1 ICIs: The Precision Immuno-Oncology for advanced Non-Small CEll Lung CancER (PIONeeR) trial.
Participants: Laurent Greillier, Florence Monville, Vanina Leca, Frédéric Vely, Stéphane Garcia, Joseph Ciccolini, Florence Sabatier, Gilbert Ferrani, Nawel Boudai, Lamia Ghezali, Marcellin Landri, Clémence Marin, Mourad Hamimed, Laurent Arnaud, Arnaud Boyer, Mélanie Karlsen, Kevin Atsou, Pauline Fleury, Clarisse Audigier Valette, Stéphanie Martinez, Hervé Pégliasco, Louisiane Lebas, Patricia Barré, Sarah Zahi, Ahmed Frika, Pierre Bory, Maryannick Le Ray, Lilian Laborde, Virginie Martin, Richard Malkoun, Marie Roumieux, Julien Mazières, Maurice Perol, Eric Vivier, Sébastien Benzekry, Jacques Fieschi, Fabrice Barlési.
Funding : This work is supported by French National Research Agency (ANR-17-RHUS-0007, a partnership of AMU, APHM, AstraZeneca, Centre Léon Bérard, CNRS, Veracyte, ImCheck Therapeutics, Innate Pharma, Inserm, Institut Paoli Calmettes and sponsored by AP HM and initiated by Marseille Immunopole. It is also supported by Plan Cancer Inserm grant number 19CM148-00 as part of the QUANTIC project.
BACKGROUND: Resistance to PD1/L1 immune checkpoint inhibitors (ICIs) in advanced NSCLC patients is observed in about 80% of individuals with no robust predictive biomarker yet. The PIONeeR trial (NCT03493581) aims to predict such resistances through a comprehensive multiparametric biomarkers analysis.
METHODOLOGY: Among the >300 advanced NSCLC patients (pts) recruited in PIONeeR, we focused on the first 137 2nd line ECOG PS0-1 patients treated with single-agent nivolumab, pembrolizumab or atezolizumab, with good performance status. Tumor tissue was collected at baseline and Pts were systematically re-biopsied at 6 weeks, and blood-sampled every cycle throughout the 24 weeks post C1D1. Response to PD1/L1 ICIs was assessed by RECIST 1.1 every 6 weeks.
Immune contexture was characterized in tumor and blood of each pt through FACS for circulating immune cell subtypes quantification, dual- and multiplex IHC / digital pathology to quantify activating & suppressive immune cells infiltrating the tumor, standardized methods for endothelial activation assessment, blood soluble factors dosage and tumor & blood WES (TMB and other). ICI plasma levels and pharmacokinetics parameters were also monitored, leading to 331 measured biomarkers in addition to routine clinical parameters. Multivariable (MV) logistic regression was used to examine the association of each biomarker (controlled by sex, age, smoking status, histological type & PD-L1 TC expression) with the risk of Early Progression (EP), i.e. within 3.5 months of treatment. Multivariable Cox regression analysis was conducted for association with PFS and OS.
SOFTWARE: This project is associated to the development of the stats_pioneer software.
RESULTS: Overall, the 137 pts were mainly male (64%), smokers (92%) and <70yrs (68%). Tumors were mainly non-squamous (79%) with >1% PDL1+ TC in 36% of the cases. 21% were still on treatment at data cut-off. Archived samples were available for 109 patients (80%) at inclusion and re-biopsy was available in 52.9% of these cases. The median follow up was 19.8 months, 22.5% of pts did not progress at data cut-off, and 85 patients present with EPs.
Tumor Cytotoxic T-cells density, especially PD1+ were lower in EP (MV OR=0.45, p=0.022); conversely, higher proportions of activated cytotoxic T-cells were observed among circulating lymphocytes in EP (MV OR=3.8, p<0.001). Among other biomarkers, Tregs (MV OR=0.44, p=0.018), 3 NK sub-populations (MV OR 0.44, p<0.05), albumin (MV OR 0.4, p<0.01) and PDL1 TC % (MV OR=0.27, p<0.01) were decreased whereas alkaline phosphatase was increased (OR=3, p=0.018). A large inter-pt variability (i.e., >65%) was observed in plasma exposures for all ICIs, with 8-10% of pts displaying trough levels below the threshold associated with target engagement. Data will be presented through unsupervised clustering algorithms and dedicated multi-modal supervised learning methods. Changes observed after 6 weeks of treatment will be analyzed to further investigate the drugs mechanisms of action.
CONCLUSION: The PIONeeR trial provides with the 1st comprehensive biomarkers’ analysis of to establish predictive models of resistance in advanced NSCLC pts treated with PD1/L1 ICIs and highlights how tumor and circulating biomarkers are complementary.
7.1.4 Tumor kinetics modeling combined to machine learning outperforms best overall response for prediction of overall survival from first-line data in head and neck squamous cell carcinoma
Participants: Kevin Atsou, Anne Auperin, Joel Guigay, Sebastien Salas, Sebastien Benzekry.
Data: Unicancer ORL group
Publication: In preparation
BACKGROUND: Prediction of overall survival (OS) and response to second-line treatment is a major challenge in the treatment of Head and Neck Squamous Cell Carcinoma (HNSCC). Tumor kinetics model-derived parameters combined to parametric survival modeling has been proposed to predict OS but quantitative comparison of predictive performances with classical (RECIST) response criteria is lacking.
METHODS: Patient data were collected from a randomized, phase 2 trial (GORTEC 2014-01 TPExtreme) aiming to compare the efficacy and safety of the TPEx (platinum-docetaxel-cetuximab) regimen against the standard of care regimen, EXTREME (platinum-fluorouracil-cetuximab). It consisted of 526 patients and 15 baseline clinical features, as well as longitudinal tumor kinetics (TK) data (sum of largest diameters). First, a double-exponential model for quantitative description of first-line TK was selected within a set of models using a training data set (70% of the full data) and mixed-effects modeling. Then, multiple machine learning (ML) algorithms for survival analysis (DeepSurv, DeepHit, CoxTime, CV CoxBoost, XGBoost, GLMBoost, Survival SVM, Pc Hazard, Cox Lasso, and Random Survival Forest) were assessed for their ability to predict individual OS, using 10 repeats of 5-folds cross-validation on the train set. Subsequently, the best model was used to predict OS on a test data set (30% of the full data).
RESULTS: The double-exponential model was able to describe individual TK data as well as inter-individual variability. It revealed a significant association between the treatment arms and the tumor growth rate. The best ML algorithm was the random survival forest. Notably, it significantly outperformed a classical proportional hazard Cox regression model (c-index of 0.69 (95% "CI" 0.68-0.7) vs 0.64 (0.63 – 0.65) on the training set, p<0.0001 and 0.68 (0.63-0.73) vs 0.61(0.54 – 0.66) on the test set, p< 0.0001). Interestingly, the most important parameters for OS prediction were the TK model parameters. Using best overall response (BOR) instead of TK significantly decreased the predictive quality of the model (c-index of 0.63 (0.62 – 0.64) vs 0.69 (0.68 - 0.7) on the training set, p<0.0001 and 0.52 (0.51 – 0.53) vs 0.685 (0.68 - 0.69) on the test set, p < 0.0001, BOR vs TK, respectively).
CONCLUSION: A combination of mechanistic modeling of first line TK and ML could accurately predict OS.
7.1.5 Modeling Anticancer Related Late Effect on Neurocognition (MARLEN study)
Participants: Xavier Muracciole, Dominique Barbolosi, Christelle Dufour, Virginie Kieffer, A. Longaud, Stéphanie Bolle, Lila Saidoun, Charles Grellois, Nicolas André, Laeticia Padovani, Dominique Jamois.
Funding and data: MARLEN Study
Publication: Abstract sent to the "3e journée nationale OncoNeuroTox" conference.
BACKGROUND: Long-term survivors of pediatric medulloblastoma present significant impairments in specific cognitive functions. In addition to radiation-related factors, the tumor itself, hydrocephalus, age, sex, and socio-economic status at diagnosis can contribute impact negatively the neurocognitive outcome. Our major objective was to create retrospectively a mathematical model evaluating the contribution of key clinical factors and cranial irradiation to impaired neurocognitive performances and their dynamic interactions.
METHODS: The cohort concerned children treated for a medulloblastoma and included in RISK-N(N°EUDRACT). We used pivot tables to select significant factors to be incorporated in complex, dynamic and non-deterministic models. We evaluated neurocognitive patient’ dynamic trajectories in terms of scholar performance or Full-Scale Intellectual Quotient (FSIQ) scores using a discrete-time Markov chains analysis. A simulator was created to describe the interactions between significant co-variates.
RESULTS: 78 patients were included with a median age of 6 years. Neurocognition outcome has been evaluated at 4 time points with school career and FSIQ score. Analysis by pivot tables revealed a difference in mean loss of FSIQ with a loss of 0.7%/year for children without hydrocephalus against 3.3%/year for children with hydrocephalus. A Markov decision analysis model was developed using four states from 1 (the best score) to 4 (the worst score). For children between 3 tot 6 years old, the probability to obtain at the last evaluation a good FSIQ (state 1 or 2) was 91% and a bad FSIQ (state 3 or 4) was 0.06% versus 79% and 21%, without or with hydrocephalus respectively. The simulator reported the dynamic interactions between the following co-variates initial FSIQ, age, sex, hydrocephalus, the socioeconomic level of the parents, and supratentorial dose or radiation therapy.
CONCLUSION: Using the Markov chain model, we elaborated a predictive mathematical tool of neurocognitive outcome which will permit to optimize the treatment of pediatric brain tumors and especially corrective medication or rehabilitation programs in hope to mitigate the impaired neurocognition.
7.2 Mechanistic modeling of metastasis
7.2.1 Mechanistic modeling of metastatic relapse in early breast cancer to investigate the biological impact of prognostic biomarkers
Participants: Célestin Bigarré, François Bertucci, Pascal Finetti, Gaëtan Macgrogan, Xavier Muracciole, Sébastien Benzekry.
Funding: Inria-Inserm Phd Grant
Publication: Under revision in ”Computer Methods and Programs in Biomedicine” (preprint 74) and AACR communication 50
BACKGROUND: Estimating the risk of metastatic relapse is a major challenge to decide adjuvant treatment options in early-stage breast cancer (eBC). To date, distant metastasis-free survival (DMFS) analysis mainly relies on classical, agnostic, statistical models (e.g., Cox regression). Instead, we propose here to derive mechanistic models of DMFS.
METHODS: The present series consisted of eBC patients who did not receive adju-vant systemic therapy from three datasets, composed respectively of 692 (Bergonié Institute), 591 (Paoli-Calmettes Institute, IPC), and 163 (Public Hospital Marseille, AP-HM) patients with routine clinical annotations. The last dataset also contained expression of three non-routine biomarkers. Our mechanistic model of DMFS relies on two mathematical parameters that represent growth () and dissemination (). We identified their population distributions using mixed-effects modeling. Critically, we propose a novel variable selection procedure allowing to: (i) identify the association of biological parameters with either , or both, and (ii) generate an optimal candidate model for DMFS prediction.
RESULTS: We found that Ki67 and Thymidine Kinase-1 were associated with , and nodal status and Plasminogen Activator Inhibitor-1 with . The predictive performances of the model were excellent in calibration but moderate in discrimination, with c-indices of 0.72 (95% CI [0.48, 0.95], AP-HM), 0.63 ([0.44, 0.83], Bergonié) and 0.60 (95% CI [0.54, 0.80], IPC).
CONCLUSIONS: Overall, we demonstrate that our novel method combining mechanistic and advanced statistical modeling is able to unravel the biological roles of clinicopathological parameters from DMFS data.
7.2.2 Practical identifiability analysis of a mechanistic model for the time to distant metastatic relapse and its application to renal cell carcinoma
Participants: Arturo Alvarez-Arenas, Wilfried Souleyreau, Andrea Emmanuelli, Lindsay Cooley, Jean-Christophe Bernard, Andreas Bikfalvi, Sebastien Benzekry.
Funding: Systems Biology of Renal Carcinoma using a mouse RCC model. National Cancer Institute (INCa) grant
Data: Laboratoire de l'Angiogénèse et du Micro-environnement des Cancers (LAMC, Inserm U1029, Bordeaux)
Publication: 11
BACKGROUND: Distant metastasis-free survival (DMFS) curves are widely used in oncology. They are classically analyzed using the Kaplan-Meier estimator or agnostic statistical models from survival analysis. Here we report on a method to extract more information from DMFS curves using a mathematical model of primary tumor growth and metastatic dissemination.
METHODS: The model depends on two parameters, α and μ, respectively quantifying tumor growth and dissemination. We assumed these to be lognormally distributed in a patient population. We propose a method for identification of the parameters of these distributions based on least squares minimization between the data and the simulated survival curve.
RESULTS : We studied the practical identifiability of these parameters and found that including the percentage of patients with metastasis at diagnosis was critical to ensure robust estimation. We also studied the impact and identifiability of covariates and their coefficients in α and μ, either categorical or continuous, including various functional forms for the latter (threshold, linear or a combination of both). We found that both the functional form and the coefficients could be determined from DMFS curves. We then applied our model to a clinical dataset of metastatic relapse from kidney cancer with individual data of 105 patients. We show that the model was able to describe the data and illustrate our method to disentangle the impact of three covariates on DMFS: a categorical one (Fuhrman grade) and two continuous ones (gene expressions of the macrophage mannose receptor 1 (MMR) and the G Protein-Coupled Receptor Class C Group 5 Member A (GPRC5a) gene). We found that all had an influence in metastasis dissemination (μ), but not on growth (α).
7.2.3 Mechanistic modeling of brain metastases in NSCLC provides computational markers for personalized prediction of outcome
Participants: Sebastien Benzekry, Pirmin Schlicke, Pascale Tomasini, Eleonore Simon.
Data: Multidisciplinary oncology and therapeutic innovation, APHM
Publication: Under review at Frontiers in Oncology, preprint 75
BACKGROUND: Intracranial progression after curative treatment of early-stage non-small cell lung cancer (NSCLC) occurs from 10 to 50% and is difficult to manage, given the heterogeneity of clinical presentations and the variability of treatments available. The objective of this study was to develop a mechanistic model of intracranial progression to predict survival following a first brain metastasis (BM) event.
METHODS: Data included early-stage NSCLC patients treated with a curative intent who had a BM as the first and single relapse site (N=31). We propose a mechanistic mathematical model to estimate the amount and sizes of (visible and invisible) BMs. The two key parameters of the model are α, the proliferation rate of a single tumor cell; and μ, the per day, per cell, probability to metastasize. The predictive value of these individual computational biomarkers was evaluated.
RESULTS: The model was able to correctly describe the number and size of metastases at the time of first BM relapse for 20 patients. Parameters α and μ were significantly associated with overall survival (OS) (HR 1.65 (1.07-2.53) p=0.0029 and HR 1.95 (1.31-2.91) p=0.0109, respectively). Adding the computational markers to the clinical ones significantly improved the predictive value of OS (c-index increased from 0.585 (95% CI 0.569-0.602) to 0.713 (95% CI 0.700-0.726), p<0.0001).
CONCLUSION: We demonstrated that our model was applicable to brain oligoprogressive patients in NSCLC and that the resulting computational markers had predictive potential. This may help lung cancer physicians to guide and personalize the management of NSCLC patients with intracranial oligoprogression.
7.3 Modeling drug delivery and pharmacokinetics
7.3.1 Macro-scale models for fluid flow in tumour tissues: impact of microstructure properties
Participants: Cristina Vaghi, Raphaelle Fanciullino, Sebastien Benzekry, Clair Poignard.
Data: Simulation and Modeling: Adaptive Response for Therapeutics in cancer (SMARTc, CRCM, Marseille)
Publication: 44
BACKGROUND: Understanding the dynamics underlying fluid transport in tumour tissues is of fundamental importance to assess processes of drug delivery.
METHODS: Here, we analyse the impact of the tumour microscopic properties on the macroscopic dynamics of vascular and interstitial fluid flow. More precisely, we investigate the impact of the capillary wall permeability and the hydraulic conductivity of the interstitium on the macroscopic model arising from formal asymptotic 2-scale techniques.
RESULTS: The homogenization technique allows us to derive two macroscale tissue models of fluid flow that take into account the microscopic structure of the vessels and the interstitial tissue. Different regimes were derived according to the magnitude of the vessel wall permeability and the interstitial hydraulic conductivity. Importantly, we provide an analysis of the properties of the models and show the link between them. Numerical simulations were eventually performed to test the models and to investigate the impact of the microstructure on the fluid transport. Future applications of our models include their calibration with real imaging data to investigate the impact of the tumour microenvironment on drug delivery.
7.3.2 Asymptotic analysis of a biphase tumor fluid flow: the weak coupling case
Participants: Cristina Vaghi, Sebastien Benzekry, Clair Poignard.
Publication: 43
BACKGROUND: The aim of this paper is to investigate the asymptotic behavior of a biphase tumor fluid flow model derived by 2-scale homogenisation techniques in recent works.
METHODS: This biphase fluid flow model accounts for the capillary wall permeability, and the interstitial avascular phase, both being mixed in the limit homogenised problem.
RESULTS: When the vessel walls become more permeable, we show that the biphase fluid flow exhibits a boundary layer that make the computation of the full problem costly and unstable. In the limit, both capillary and interstitial pressures coincide except in the vicinity of the boundary where different boundary conditions are applied. Thanks to a rigorous asymptotic analysis, we prove that the solution to the full problem can be approached at any order of approximation by a monophasic model with appropriate boundary conditions on the tumor boundary and appropriate correcting terms near the boundary are given. Numerical simulations in spherical geometry illustrate the theoretical results.
7.3.3 Cetuximax: maximizing Cetuximab efficacy in head and neck cancer patients through PK/PD modeling
Participants: Clémence Marin, Mourad Hamimed, Joseph Ciccolini, Sébastien Salas.
Funding and data: Merck Serono, APHM, GPCO-Unicancer network
Publication: IATDMCT, Rome, 2021 and AACR 2022
BACKGROUND: anti-EGFR cetuximab is a pivotal mAb in head and neck cancer patients in association with chemotherapy. Therapeutic window of cetuximab and possible room for adaptive dosing strategies are unknown.
METHODS: In this multicentric clinical trial registered as NCT4218136 (P.I.: Pr Sebastien Salas), pharmacokinetics and pharmacodynamics of cetuximab will be both assessed so as to help determining the metrics (e.g., trough levels, Cmax, AUC) associated with both efficacy and toxicity of cetuximab in head and neck cancer patients. Once the therapeutic window is established, an adaptive dosing algorithm will be propose to customize drug dosing and/or scheduling.
SOFTWARE: PK/PD modeling and adaptive dosing algorithm are performed on MonoLix (Lixoft France)
RESULTS: Early Results suggest that 34 µg/ml could be the threshold associated with efficacy upon Recist criteria. Conversely, it is not possible yet to identify exposure levels associated with severe toxicities. A large inter-individual variability in PK parameters and exposure levels has been observed (i.e., > 60%), calling for developing therapeutic drug monitoring strategies to check exposure levels. The inclusion phase is nearly completed with a total of 110 patients included as of January 2023. PK analysis for Cetuximab plasma levels and PK/PD analysis should start in 2023.
CONCLUSION: the early results of this study confirm that PK/PD relationships with cetuximab plus large variability observed in exposure levels warrants the need for customizing dosing to ensure a maximum efficacy.
7.3.4 MOVIE: Metronomic oral vinorelbine plus anti-PD-L1/anti-CTLA4 immunotherapy in patients with advanced solid tumours
Participants: Mourad Hamimed, Loic Osanno, Joseph Ciccolini, Céline Vicier, Anthony Goncalves.
Funding and data: INCa PHRC-K (recipient: Anthony Goncalves, IPC)
Publication: 45
BACKGROUND: The MOVIE trial is a phase-I trial designed to determine the best dosing of metronomic Vinorelbine to be associated with immune checkpoint inhibitors durvalumab and tremelimumab, prior to start a Phase 2 for efficacy.
METHODS: This is a clinical trial registred as NCT03518606 (PI: Pr Anthony Goncalves). MOVIE is a multicohort phase I/II study examining the combination of anti-PD-L1 durvalumab (Durv; 1500 mg IV Q4W) plus anti-CTLA tremelimumab (Trem; 75 mg IV Q4W) with metronomic vinorelbine (MVino; 20-40 mg orally daily) in various AST resistant to conventional therapies. The primary objective of the phase I part was to determine the maximum tolerated dose (MTD) and recommended dose for phase II (RP2D).
SOFTWARE: PK modeling was investigated on MonoLix (Lixoft France).
RESULTS: Results showed a marked variability between patients on Vinorelbine PK parameters. MTD was not reached. The safety profile of the combination was manageable and consistent with previous reports of Trem + Durv or MVino. Phase II is currently ongoing in BC, PC, CC, HNC, and miscellaneous cohorts.
CONCLUSION: Dose level 2 (MVino 40 mg, Durv 1500 mg, Trem 75 mg) was selected as RP2D to be further tested.
7.3.5 Determination of the therapeutic window of cisplatin in head and neck cancer patients
Participants: Celeste Cauvin, Laurent Bourguignon, Laure Carriat, Abel Mence, Pauline Ghipponi, Sébastien Salas, Joseph Ciccolini.
Funding and data: APHM real-world study under the MR004 status.
Publication: 19
BACKGROUND and METHODS: We analyzed the exposure-effect relationships of 80 adult patients with head and neck cancers and treated with standard Cisplatin-based regimen administered as three-hour infusion. Individual pharmacokinetics (PK) parameters of Cisplatin were identified using a Bayesian approach. Nephrotoxicity and ototoxicity were considered as typical Cisplatin-related toxicities according to Common Terminology Criteria for Adverse Events (CTCAE) standards. Efficacy was evaluated based upon Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Up to nine different machine-learning algorithms were tested to decipher the exposure-effect relationships with Cisplatin.
SOFTWARE: PK modeling was investigated on Kinetic Pro (MIIPS France). ML analysis were performed on R.
RESULTS: The generalized linear model was the best algorithm with an accuracy of 0.71, a recall of 0.55 and a precision of 0.75. Among the various metrics for exposure (i.e., maximal concentration (Cmax), area-under-the-curve (AUC), trough levels), Cmax, comprising a range between 2.4 and 4.1 µg/mL, was the best one to be considered. When comparing a consequent, model-informed dosage with the standard dosage in 20 new patients, our strategy would have led to a reduced dosage in patients who would eventually prove to have severe toxicities while increasing dosage in patients with progressive disease.
CONCLUSION: Determining a target Cmax could pave the way for PK-guided precision dosage with Cisplatin given as three-hour infusion.
7.3.6 Impact of pharmacogenetics on variability in exposure to oral vinorelbine among pediatric patients: a model-based population pharmacokinetic analysis
Participants: Mourad Hamimed, Pierre Leblond, Nicolas André, Joseph Ciccolini.
Funding and data: INCa.
Publication: 28
BACKGROUND: Better understanding of pharmacokinetics of oral vinorelbine (VNR) in children would help predicting drug exposure and, beyond, clinical outcome. Here, we have characterized the population pharmacokinetics of oral VNR and studied the factors likely to explain the variability observed in VNR exposure among young patients.
METHODS: We collected blood samples from 36 patients (mean age 11.6 years) of the OVIMA multicentric phase II study in children with recurrent/progressive low-grade glioma. Patients received 60 mg/m2 of oral VNR on days 1, 8, and 15 during the first 28-day treatment cycle and 80 mg/m2, unless contraindicated, from cycle 2-12. Population pharmacokinetic analysis was performed using nonlinear mixed-effects modeling within the Monolix® software. Fifty SNPs of pharmacokinetic-related genes were genotyped. The influence of demographic, biological, and pharmacogenetic covariates on pharmacokinetic parameters was investigated using a stepwise multivariate procedure.
SOFTWARE: PK modeling was investigated on Monolix software (Lixoft) plus R packages.
RESULTS: A three-compartment model, with a delayed double zero-order absorption and a first-order elimination, best described VNR pharmacokinetics in children. Typical population estimates for the apparent central volume of distribution (Vc/F) and elimination rate constant were 803 L and 0.60 h-1, respectively. Following covariate analysis, BSA, leukocytes count, and drug transport ABCB1-rs2032582 SNP showed a dramatic impact on Vc/F. Conversely, age and sex had no significant effect on VNR pharmacokinetics.
CONCLUSION: Beyond canonical BSA and leukocytes, ABCB1-rs2032582 polymorphism showed a meaningful impact on VNR systemic exposure. Simulations showed that the identified covariates could have an impact on both efficacy and toxicity outcomes. Thus, a personalized dosing strategy, using those covariates, could help to optimize the efficacy/toxicity balance of VNR in children.
7.3.7 Revisiting metronomic vinorelbine with mathematical modelling: a Phase I trial in lung cancer
Participants: Laure Deyme, Diane-Charlotte Imbs, Joseph Ciccolini, Dominique Barbolosi.
Funding and data: APHM and Pierre Fabre.
Publication: 12
BACKGROUND: A phase Ia/Ib trial of metronomic oral vinorelbine (MOV) driven by a mathematical model was performed in heavily pretreated metastatic Non-Small Cell Lung Cancer or Pleural Mesothelioma patients. Disease Control Rate, progression free survival, toxicity and PK/PD were the main endpoints. METHODS: Best MOV scheduling was selected using a simplified phenomenological, semi-mechanistic model with a total weekly dose of 150-mg vinorelbine. Computation of individual PK parameters was performed using a population approach.
SOFTWARE: PK modeling was investigated on Monolix software (Lixoft) plus R packages.
RESULTS: The mathematical model proposed the following metronomic schedule for a 150-mg weekly dose of vinorelbine: 60 mg D1, 30 mg D2, 60 mg D4. A total of 37 heavily pre-treated patients (30 evaluable) were enrolled. Grade III/IV neutropenia was observed in 30% of the patients. Median PFS was 11 weeks. Disease Control Rate was 73% (i.e.; 13% partial response and 60% stable disease). A large variability in drug exposure (AUC0-24 h: 53%) and PK parameters (Cl: 83%) were observed among patients. Simulated trough levels after D2 and D4 showed similarly 56-73% variability among patients. Drug exposure was not associated with efficacy, but neutropenia was more frequent in patients with AUC > 250 ng/ml.h. Tumor burden, performance status and neutrophils-to-lymphocyte ratio (NLR) were associated with PFS, suggesting that MOV would be indicated in selected patients. We built a composite score to predict efficacy, mixing baseline tumor size and NLR showing 84% sensitivity and 75% specificity.
CONCLUSION: MOV was characterized by important variability in drug exposure among patients. However, and despite being all heavily pre-treated, 73percent of disease control rate and 11 weeks PFS were achieved with manageable toxicities. PK/PD relationships yielded conflicting results depending on the initial tumor burden and BSA, suggesting that patients should be carefully selected prior to be scheduled for metronomic regimen. Possible role NLR could play as a predictive marker suggests immunomodulating features with MOV.
7.3.8 Mathematical tools for tumor volume monitoring in breast cancer bearing mice
Participants: Anne Rodallec, Cristina Vaghi, Joseph Ciccolini, Raphaelle Fanciullino, Sebastien Benzekry.
Funding and data: No funding. Data from SMARTc and COMPO
Publication: 38
BACKGROUND: Although recent regulations improved conditions of laboratory animals, their use remains essential in cancer research to determine treatment efficacy. In most cases, such experiments are performed on xenografted animals for which tumor volume is mostly estimated from caliper measurements. However, many formulas have been employed for this estimation and no standardization is available yet.
METHODS: Using previous animal studies, we compared all formulas used by the scientific community in 2019. Data were collected from 93 mice orthotopically xenografted with human breast cancer cells. All formulas were evaluated and ranked based on correlation and lower mean relative error. They were then used in a Gompertz quantitative model of tumor growth.
SOFTWARE: data were analyzed on R-4.0.3
RESULTS: Seven formulas for tumor volume estimation were identified and a statistically significant difference was observed among them (ANOVA test, p < 2.10-16), with the ellipsoid formula (1/6 π × L × W × (L + W)/2) being the most accurate (mean relative error = 0.272 ± 0.201). This was confirmed by the mathematical modeling analysis where this formula resulted in the smallest estimated residual variability. Interestingly, such result was no longer valid for tumors over 1968 ± 425 mg, for which a cubic formula (L x W x H) should be preferred.
CONCLUSION: When considering that tumor volume remains under 1500mm3, to limit animal stress, improve tumor growth monitoring and go toward mathematic models, the following formula 1/6 π × L × W x (L + W)/2 should be preferred.
7.3.9 Semi-mechanistic modeling to show the impact of structural modifications on radiolabeled-dendrimers pharmacokinetics
Participants: Jessica Ou, Béatrice Louis, Vincent Nail, Laure Balasse, Ahlem Bouhlel, Adrien Chabert, Tom Roussel, Ling Peng, Philippe Garrigue, Florence Gattacceca.
Funding: ED62 PhD Grant
Data: CERIMED (Aix-Marseille University)
INTRODUCTION: Nanosizing promotes the penetration of substances into solid tumors via the enhanced permeability and retention effect. Hence, CERIMED and CINaM teams designed a supramolecular [68Ga]Ga-radiolabeled self-assembling dendrimer (A) for tumor imaging. Dendrimer A was first optimized through chemical modifications to lower exposure of healthy organs, leading to 4 new dendrimer formulations (B,C,D,E). The best dendrimer was then selected and functionalized with RGD at different ratios, generating 2 other dendrimers (F,G). To understand the impact of structural modifications on the dendrimers biodistribution, a pharmacokinetic (PK) study was performed.
MATERIALS AND METHODS: Two-hour-long microPET/CT and gamma-counting biodistribution data of each radiolabeled dendrimer were collected from healthy Swiss mice (n=6). Non-compartmental analysis (NCA) and population compartmental analysis (PopCA) were achieved respectively with PKanalix and Monolix softwares. NCA was used to describe exposure and main PK parameters in blood and organs for each formulation. PopCA was based on a semi-mechanistic model constituted of 10 compartments and considering both renal and hepatic eliminations. The semi-mechanistic model allowed to evaluate the impact of dendrimer type on transfer rate constants between compartments especially on elimination mechanisms.
RESULTS: Functionalization with RGD seemed to not impact pharmacokinetics, and dendrimers F and G behaved similarly to dendrimer E based on NCA. Dendrimer E displayed the lowest systemic exposure (area under concentration versus time curve (AUC) of 38 %ID/mL.h versus 71-138 %ID/mL.h), and consistently the highest clearance CL (2.66 mL/h versus 0.83-1.48 mL/h) which could be attributed to a more efficient renal elimination as suggested by bladder data. The volume of distribution (Vd) was also higher than for other dendrimers (8.00 mL versus 3.95-5.65 mL), suggesting a wider distribution in the organism, in line with the higher concentrations observed at the earliest time points in the main organs. AUCs in the main organs and especially in the liver, were contrarily lower for dendrimer E, due to a shorter terminal half-life (HL) in organs. Dendrimer E also displayed a short blood HL (2.21 h versus 1.99-4.36 h). The semi-mechanistic model estimated rate transfer constants between compartments with a good precision. The semi-mechanistic model showed that dendrimer F and G were less long retained in the liver compared to the other dendrimers, with higher liver elimination constants meaning that functionalization would have an impact on liver elimination. Surprisingly, no statistical difference was found for dendrimer E on rate transfer constants but the low exposure in liver could be explained by a low affinity to liver (AUCliver/AUCblood ratio) and a rapid renal clearance. Consistently, dendrimer E displayed a lower transfer rate constant from kidneys to blood compared to reference dendrimer A (14.47 h-1 versus 29.72 h-1) and a higher transfer rate constant from kidneys to bladder (4.07 h-1 versus 1.94 h-1), confirming a better renal excretion through glomerular filtration. Moreover, kinetics in kidneys and bladder were different following the type of dendrimer suggesting different renal elimination mechanisms. Therefore, exit rate constant from bladder to blood was added to explain the early saturation in bladder and was significantly higher for dendrimers A,B, and C than for dendrimer D,E,F and G (1.15 h-1 versus 0.18 h-1) which could be ascribed to bladder resorption.
CONCLUSION: PK studies allowed the selection of an optimal dendrimer (dendrimer E) displaying the lowest exposure of healthy organs and the fastest clearance for functionalization studies. PopCA provided a mechanistic understanding of the differences in PK behavior. Dendrimers with shorter carbon chain length seemed to be cleared faster from the organism via hepatic and renal eliminations. Functionalization with RGD seemed to improve hepatic elimination. The semi-mechanistic model is also a first step toward the development of a physiologically-based pharmacokinetic model, which could be a useful tool to predict PK behavior of dendrimers depending on their chemical structure for nanoparticle optimization.
8 Bilateral contracts and grants with industry
8.1 Research contracts
D-LIGHT
- Partner: Roche
- Title: Tumor growth inhibition modeling coupled with machine learning for early response prediction and identification of common features of response, to optimize combination therapies in Oncology
- Funding: 76.5 k€
- Duration: Jan 2021 - Apr 2022
- Principal investigator: S. Benzekry
NanoImmuno
- Partner: Institut Roche France and Genentech US
- Title: NanoImmuno: a nanoparticle to harness tumor immunity in her2+ breast cancer
- Funding: 80 k€
- Duration: Jan 2020 - March 2023
- Principal investigator: Raphaelle Fanciullino (Senior), Anne Rodallec (Junior)
8.2 Clinical trials
CetuxiMAX
- Registration: NCT4218136
- Partner: Merck Serono
- Title: Maximizing Cetuximab efficacy in head and neck cancer patients through PK/PD modeling
- Funding: 40 k€
- Duration: 2020 - 2023
- Principal investigator: Sébastien Salas
MOVIE
- Registration: NCT03518606
- Partner: Astra Zeneca and INCa
- Title: Metronomic Oral Vinorelbine Plus Anti-PD-L1/Anti-CTLA4 ImmunothErapy in Patients With Advanced Solid Tumours
- Funding: 500 k€
- Duration: 2019-2022
- Principal investigator: Anthony Goncalves (IPC)
- Compo investigator: Joseph Ciccolini
MOIO
- Registration: NCT05078047
- Partner: BMS and INCa
- Title: Study Comparing the Standard Administration of IO Versus the Same IO Administered Each 3 Months in Patients With Metastatic Cancer in Response After 6 Months of Standard IO
- Funding: 500 k€
- Duration: 2020-2024
- Principal investigator: Gwenaelle Gravis (IPC)
- Compo investigator: Joseph Ciccolini
VIDAZAML
- Registration: EudraCT 2020-A02042-37
- Partner: APHM and CAL Nice
- Title: Role of CDA and dCK metabolic enzymes in clinical response of patients treated with azacitidine for blood disorders
- Funding: 80 k€ (APHM)
- Duration: 2021-2023
- Principal investigator: Geoffroy Venton - Compo Investigator: Raphaelle Fanciullino
Morpheus-Lung
- Registration: Eudract 2017-001267-21 / NCT03337698
- Partner: Hoffmann-La Roche
- Title: A Study to Evaluate Efficacy and Safety of Multiple Immunotherapy-Based Treatment Combinations in Patients with Metastatic Non-Small Cell Lung Cancer
- Duration: 2018-2023
- Compo investigator: Laurent Greillier
PDC-LUNG-101
- Registration: Eudract 2018-002382-19 / NCT03970746
- Partner: PDC*line Pharma SAS
- Title: Safety, immunogenicity and preliminary clinical activity study of PDC*lung01 cancer vaccine in NSCLC
- Duration: 2022-2024
- Principal investigator: Johan Vansteenkiste, Prof KU Leuven
- Compo investigator: Laurent Greillier
NCT03840915
- Registration: Eudract 2018-004040-28 / NCT03840915
- Partner: EMD Serono Research and Development Institute, Inc., Merck KGaA
- Title: A Phase Ib/II, Open-Label Study of M7824 in Combination With Chemotherapy in Participants With Stage IV Non-small Cell Lung Cancer
- Duration: 2019-2022
- Compo investigator: Laurent Greillier
NCT03708328
- Registration: Eudract 2018-000982-35 / NCT03708328
- Partner: Hoffmann-La Roche
- Title: A Dose Escalation and Expansion Study of RO7121661, a PD-1/TIM-3 Bispecific Antibody, in Participants With Advanced and/or Metastatic Solid Tumors
- Duration: 2018-2023
- Compo investigator: Laurent Greillier
PROPEL
- Registration: Eudract 2019-003474-35 / NCT03138889
- Partner: Nektar Therapeutics
- Title: Bempegaldesleukin and Pembrolizumab With or Without Chemotherapy in Locally Advanced or Metastatic Solid Tumors
- Duration: 2017-2024
- Compo investigator: Laurent Greillier
TRIDENT-1
- Registration: Eudract 2016-003616-13 / NCT03093116
- Partner: Turning Point Therapeutics, Inc.
- Title: A Study of Repotrectinib (TPX-0005) in Patients With Advanced Solid Tumors Harboring ALK, ROS1, or NTRK1-3 Rearrangements
- Duration: 2017-2023
- Principal investigator: Shanna Stopatschinskaja, Dan Liu
- Compo investigator: Laurent Greillier
NCT04721015
- Registration: Eudract 2020-004953-57 / NCT04721015
- Partner: AbbVie
- Title: Study of Intravenous (IV) ABBV-637 Alone or in Combination With IV Docetaxel/Osimertinib to Assess Adverse Events and Change in Disease Activity in Adult Participants With Relapsed/Refractory (R/R) Solid Tumors
- Duration: 2021-2026
- Compo investigator: Laurent Greillier
PIONeeR
- Registration: NCT03493581
- Partner: APHM, HalioDx, Innate Pharma, AMU
- Title: Precision Immuno-Oncology for Advanced Non-small Cell Lung Cancer Patients With PD-1 ICI Resistance
- Duration: 2018-2022
- Principal investigator: Laurent Greillier, Jean-Olivier Arnaud
ELDERLY
- Registration: NCT03977194
- Partner: Intergroupe Francophone de Cancerologie Thoracique
- Title: Atezolizumab in Elderly Patients With Advanced Non-Small-Cell Lung Cancer and Receiving Carboplatin Paclitaxel Chemotherapy
- Duration: 2019-2023
- Principal investigator: Elisabeth QUOIX, Céline MASCAUX
- Compo investigator: Laurent Greillier
NCT01817192
- Registration: NCT01817192
- Partner: Razor Genomics
- Title: Adjuvant Chemotherapy in Patients With Intermediate or High Risk Stage I or Stage IIA Non-squamous Non-Small Cell Lung Cancer
- Duration: 2020-2025
- Principal investigator: David R Spigel, Sarah Cannon
- Compo investigator: Laurent Greillier
SAVIMMUNE
- Registration: NCT04108026
- Partner: Intergroupe Francophone de Cancerologie Thoracique
- Title: Immunotherapy in Patient With Poor General Condition
- Duration: 2020-2023
- Principal investigator: Valérie GOUNANT, Michael DURUISSEAUX
- Compo investigator: Laurent Greillier
NCT04042558
- Registration: NCT04042558
- Partner: Centre Francois Baclesse
- Title: A Study Evaluating Platinum-Pemetrexed-Atezolizumab (+/-Bevacizumab) for Patients With Stage IIIB/IV Non-squamous Non-small Cell Lung Cancer With EGFR Mutations, ALK Rearrangement or ROS1 Fusion Progressing After Targeted Therapies
- Duration: 2019-2024
- Compo investigator: Laurent Greillier
NIPINEC
- Registration: NCT03591731
- Partner: Intergroupe Francophone de Cancerologie Thoracique, Federation Francophone de Cancerologie Digestive, GERCOR - Multidisciplinary Oncology Cooperative Group
- Title: Nivolumab +/- Ipilimumab in Patients With Advanced, Refractory Pulmonary or Gastroenteropancreatic Poorly Differentiated Neuroendocrine Tumors (NECs)
- Duration: 2019-2023
- Principal investigator: Nicolas GIRARD, Thomas WALTER
- Compo investigator: Laurent Greillier
RESILIENT
- Registration: NCT03088813
- Partner: Ipsen
- Title: Study of Irinotecan Liposome Injection (ONIVYDE®) in Patients With Small Cell Lung Cancer
- Duration: 2018-2022
- Compo investigator: Laurent Greillier
Canopy-A
- Registration: NCT03447769
- Partner: Novartis Pharmaceuticals
- Title: Study of Efficacy and Safety of Canakinumab as Adjuvant Therapy in Adult Subjects With Stages AJCC/UICC v. 8 II-IIIA and IIIB (T>5cm N2) Completely Resected Non-small Cell Lung
- Duration: 2018-2027
- Compo investigator: Laurent Greillier
NCT04350463
- Registration: NCT04350463
- Partner: Celgene
- Title: A Safety and Efficacy Study of CC-90011 in Combination With Nivolumab in Subjects With Advanced Cancers
- Duration: 2020-2024
- Compo investigator: Laurent Greillier
TROPION-LUNG01
- Registration: NCT04656652
- Partner: Daiichi Sankyo, Inc., AstraZeneca
- Title: Study of DS-1062a Versus Docetaxel in Previously Treated Advanced or Metastatic Non-small Cell Lung Cancer Without Actionable Genomic Alterations
- Duration: 2020-2024
- Compo investigator: Laurent Greillier
MERMAID-1
- Registration: NCT04385368
- Partner: AstraZeneca
- Title: Phase III Study to Determine the Efficacy of Durvalumab in Combination With Chemotherapy in Completely Resected Stage II-III Non-small Cell Lung Cancer (NSCLC)
- Duration: 2020-2026
- Principal investigator: Solange Peters, Charles Swanton
- Compo investigator: Laurent Greillier
NCT03840902
- Registration: NCT03840902
- Partner: EMD Serono Research and Development Institute, Inc., Merck KGaA
- Title: M7824 With cCRT in Unresectable Stage III Non-small Cell Lung Cancer (NSCLC)
- Duration: 2019-2023
- Compo investigator: Laurent Greillier
CARMEN-LC03
- Registration: NCT04154956
- Partner: Sanofi
- Title: SAR408701 Versus Docetaxel in Previously Treated, Carcinoembryonic Antigen-related Cell Adhesion Molecule 5 (CEACAM5) Positive Metastatic Non-squamous Non-small Cell Lung Cancer Patients
- Duration: 2020-2024
- Compo investigator: Laurent Greillier
SKYSCRAPER-03
- Registration: NCT04513925
- Partner: Hoffmann-La Roche
- Title: A Study of Atezolizumab and Tiragolumab Compared With Durvalumab in Participants With Locally Advanced, Unresectable Stage III Non-Small Cell Lung Cancer (NSCLC)
- Duration: 2020-2027
- Compo investigator: Laurent Greillier
NCT03899155
- Registration: NCT03899155
- Partner: Bristol-Myers Squibb
- Title: Pan Tumor Nivolumab Rollover Study
- Duration: 2019-2025
NCT03798535
- Registration: NCT03798535
- Partner: AstraZeneca
- Title: First Real-world Data on Unresectable Stage III NSCLC Patients Treated With Durvalumab After Chemoradiotherapy
- Duration: 2018-2023
- Compo investigator: Laurent Greillier
IMbrella A
- Registration: NCT03148418
- Partner: Hoffmann-La Roche
- Title: A Study in Participants Previously Enrolled in a Genentech- and/or F. Hoffmann-La Roche Ltd-Sponsored Atezolizumab Study
- Duration: 2017-2030
- Compo investigator: Laurent Greillier
BEAT-meso
- Registration: NCT03762018
- Partner: European Thoracic Oncology Platform, Hoffmann-La Roche
- Title: Bevacizumab and Atezolizumab in Malignant Pleural Mesothelioma
- Duration: 2019-2024
- Principal investigator: Enriqueta Felip, Sanjay Popat
- Compo investigator: Laurent Greillier
SPECTA
- Registration: NCT02834884
- Partner: European Organisation for Research and Treatment of Cancer - EORTC
- Title: Screening Cancer Patients for Efficient Clinical Trial Access
- Duration: 2017-2026
- Principal investigator: Vassilis Golfinopoulos
- Compo investigator: Laurent Greillier
TROPION-Lung05
- Registration: EudraCT 2020-002774-27
- Partner: Daiichi Sankyo, Inc.
- Title: Phase 2 Study of DS-1062a in Advanced or Metastatic Non-small Cell Lung Cancer with Actionable Genomic Alterations
- Starting year: 2021
- Compo investigator: Laurent Greillier
SKYSCRAPER-06
- Registration: NCT04619797
- Partner: Hoffmann-La Roche
- Title: A Study of Tiragolumab in Combination With Atezolizumab Plus Pemetrexed and Carboplatin/Cisplatin Versus Pembrolizumab Plus Pemetrexed and Carboplatin/Cisplatin in Participants With Previously Untreated Advanced Non-Squamous Non-Small Cell Lung Cancer
- Duration: 2020 - 2025
- Compo investigator: Laurent Greillier
PERSEE
- Registration: EudraCT 2020-002626-86
- Partner: CHRU de Brest
- Title: A trial comparing the pembrolizumab platinum based chemotherapy combination with pembrolizumab monotherapy in first line treatment of non small-cell lung cancer (NSCLC) patients
- Starting year: 2020
- Principal investigator: Renaud Descourt, Chantal Decroisette, Christos Chouaid
- Compo investigator: Laurent Greillier
SAPPHIRE
- Registration: EudraCT 2019-001043-41
- Partner: Mirati Therapeutics, Inc.
- Title: A Randomized Phase 3 Study of Sitravatinib in Combination with Nivolumab Versus Docetaxel in Patients with Advanced Non-Squamous Non-Small Cell Lung Cancer with Disease Progression On or After Platinum-Based Chemotherapy and Checkpoint Inhibitor Therapy
- Starting year: 2020
- Compo investigator: Laurent Greillier
Nivothym
- Registration: EudraCT 2015-005504-28
- Partner: European Organisation for Research and Treatment of Cancer
- Title: Single-arm, multicenter, phase II study of immunotherapy in patients with type B3 thymoma and thymic carcinoma previously treated with chemotherapy
- Starting year: 2017
- Principal investigator: Nicolas Girard, Solange Peters
- Compo investigator: Laurent Greillier
9 Partnerships and cooperations
9.1 National initiatives
QUANTIC
-
Title:
QUANTitative modeling combined to statistical learning to understand and predict resistance to Immune-checkpoint inhibition in non-small cell lung Cancer
-
Partner Institutions:
- AP-HM, Marseille
- Inserm, Marseille
- Aix-Marseille University, Marseille
- Veracyte, Marseille
- InnatePharma, Marseille
-
Date/Duration:
2019 - 2023
-
Funding:
338 k€, Inserm Plan Cancer MIC
-
Principal investigator:
S. Benzekry
-
COMPO members involved:
L. Greillier, M. Karlsen, P. Dufosse.
PIONeeR - biomarkers
-
Title:
Precision Immuno-Oncology for advanced Non-small cell lung cancer patients with PD-1 ICI Resistance
-
Partner Institutions:
- AP-HM, Marseille
- 13 national cancer centers, including Centre Lyon Bérard and IUCT in Toulouse
- Marseille Immmunopôle (immuno-monitoring platform)
- Vasculo-monitoring platform of AP-HM
- Inserm
- Aix-Marseille University
- Veracyte, Marseille
- InnatePharma, Marseille
-
Date/Duration:
2018 - 2023
-
Funding:
8 M€, ANR
-
Principal investigator:
F. Barlesi (IGR)
-
COMPO members involved:
S. Benzekry, J. Ciccolini, L. Greillier, P. Dufosse, M. Hamimed, M. Karlsen, S. Marolleau, C. Marin.
THERMONANO
-
Title:
Nanoassemblies for the subcutaneous self-administration of anticancer drugs
-
Partner Institution:
- Institut Galien Paris-Saclay (UMR CNRS 8612)
-
Date/Duration:
2019 - 2024
-
Funding:
1.8 M€, ERC
-
Principal investigator:
J. Nicolas (Institut Galien, Paris-Sud)
-
COMPO members involved:
A. Rodallec, S. Benzekry, S. Marolleau.
PAIR PANCREAS
-
Title:
Determination of the sensitivity to anticancer drugs of pancreatic cancers: development of a platform for precision medicine based upon patients organoids.
-
Partner Institution:
- Pancreas Cancer Team (CRCM)
-
Date/Duration:
2019 - 2022
-
Funding:
500 K€, INCa
-
Principal investigator:
N. Dusetti (CRCM)
-
COMPO members involved:
J. Ciccolini, S. Elkaoutari
COMPLICITY
-
Title:
COMPutationaL tools for NanoBooster In Cancer ImmunoTherapY
-
Partner Institution:
- Pancreas Cancer Team (CRCM)
-
Date/Duration:
2022-2024
-
Funding:
25 K€, Amidex
-
Principal investigator:
A. Rodallec (CRCM)
-
COMPO members involved:
A. Rodallec, F. Gattacceca, C. Perez
PEMBOV
-
Title:
Pembrolizumab in Combination With Bevacizumab and Pegylated Liposomal Doxorubicin in Patients With Ovarian Cancer
-
Partner Institution:
- Institut Gustave Roussy (IGR)
-
Date/Duration:
2021-2024
-
Funding:
300 K€, INCa
-
Principal investigator:
J. Michels (IGR)
-
COMPO members involved:
J. Ciccolini, M. Hamimed
PEMEBRAIN
-
Title:
Intrathecal administration of low dose Pemetrexed in patients with brain metastasis: pharmacokinetics exploration
-
Partner Institution:
- Assistance Publique Hôpitaux de paris(APHP)
-
Date/Duration:
2022-2023
-
Funding:
APHP
-
Principal investigator:
P. Decq (APHP)
-
COMPO members involved:
J. Ciccolini, C. Boéri
10 Dissemination
10.1 Promoting scientific activities
10.1.1 Scientific events: organisation
Member of the organizing committees
- A. Rodallec: CRS BNLF ECS Meeting, Avr. 2022
- J. Ciccolini: PAMM EORTC Winter Meeting, Florence Italy, Dec. 2022
- F. Gattacceca: CMP PBPK workshop, Marseille, Dec. 2022
10.1.2 Scientific events: selection
Member of the conference program committees
- A. Rodallec: CRS BNLF ECS Meeting, Avr. 2022
- J. Ciccolini: PAMM EORTC Winter Meeting, Dec. 2022
- J. Ciccolini: IATDMCT Conference, Anticancer Drug Steering Committee, Sep. 2022
10.1.3 Journal
Member of the editorial boards
- S. Benzekry, Mathematical Biosciences
- J. Ciccolini, Cancer Chemotherapy & Pharmacology
- J. Ciccolini, British Journal of Cancer Special Issue "Advances in check point inhibitor use through pharmacological endpoints"
- F Gattacceca: Cancer Chemotherapy and Pharmacology
- A. Rodallec, Pharmaceutics Section "Pharmacokinetics and Pharmacodynamics"
Reviewer - reviewing activities
- S. Benzekry: Cancer Research, Clinical Pharmacology and Therapeutics: Pharmacometrics and Systems Pharmacology, PLoS Computational Biology, Respiratory Medicine, Maths In Action.
- J. Ciccolini: Pharmaceutics, Cancer Chemotherapy & Pharmacology, Pharmaceuticals, ESMO open, Annals of Oncology, British Journal of Clinical Pharmacology, Pharmaceutical Research, Cancer Letters, Journal of Pharmaceutical and Biomedical Analysis, Critical Reviews in Oncology/Hematology, Acta Oncologica, Clinical Cancer Research, Frontiers in Pharmacology, Journal of Chromatography B, Clinical Pharmacokinetics
- F Gattacceca: Cancer Chemotherapy and Pharmacology
- A. Rodallec: Pharmaceutics, Cancer Chemotherapy and Pharmacology
10.1.4 Invited talks
- S. Benzekry, May, 2022. 8th international conference on Systems Biology of Mammalian Cells: From Systems Medicine towards Digital Health, Heidelberg, Germany
- S. Benzekry, April, 2022. Quantitative systems pharmacology conference, Leiden, The Netherlands
- S. Benzekry, March, 2022. MI Day " Immunotherapies & Lung Cancer : predictive biomarkers from The PIONeeR Project ", Marseille, France
- S. Benzekry, February, 2022. Seminar in the bioinformatics group of the CRCM, Marseille, France.
- S. Benzekry January, 2022. Workshop on tissue growth and movement. Poincaré institute, Paris, France
- C. Bigarré, September, 2022. ECMTB 2022: The 12th European Conference on Mathematical and Theoretical Biology, Heidelberg, Germany
- J. Ciccolini December, 2022. "TDM of Biologics in oncology: hype or hope?", PAMM-EORTC 47th Winter Meeting, Florence, Italy.
- J. Ciccolini November, 2022. "Model-informed and PK-guided dosing in the era of immunotherapy", APSA-ASCEPT Joint Conference, Perkins Institute , Perth, Australia.
- J. Ciccolini November, 2022. "Place des anti-BRAF dans le mCRC BRAF-mut: aspects pharmacologiques", Cour Saint Paul Oncologie Digestive, Nice, France.
- J. Ciccolini November, 2022. "Pharmacology, pharmacocinétique et relations PK/PD des anti-CDK4/6 dans le cancer du sein", Pfizer Internal Seminar, Paris, France.
- J. Ciccolini October, 2022. "Pharmacometrics of immune checkpoint Inhibitors", Eurobiomed Workshop, Marseille, France.
- J. Ciccolini October, 2022. "Cancer nanomedicine - from the bench to the bedside", European Cost Action Nanos2Clinics,Sapienza University, Roma Italy.
- J. Ciccolini September, 2022. "Stratégies anti-BRAF anti-MEK dans le mélanome: aspects pharmacocinétiques et pharmacodynamiques.", Journées Horizon Mélanome, Paris, France.
- J. Ciccolini September, 2022. "Dose individualisation of TKIs" Pharmacokinetics Session, European Society for Medical Oncology (ESMO)Meeting 2022, Paris, France
- J. Ciccolini September, 2022. "Model informed precision dosing of biotherapeutics", Biologics Session, International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) Meeting 2022, Prague, Czeck Republic
- J. Ciccolini June, 2022 "Pharmacokinetics of mAbs in oncology: any room for TDM and Pharmacometrics? ", European Association for Clinical Pharmacology & Therapeutics (EACPT) 15th Congress, Athens, Greece.
- X. Muracciole, November, 2022. Journée SFCE 2022, Nice, France
- X. Muracciole, june, 2022. Forum en oncon-urologie (2e édition), Marseille, France
- R. Fanciullino January, 2022 "L’immuno-Oncologie au delà de l'hôpital", 5ème Journée des JSIC Paris, France.
10.1.5 Leadership within the scientific community
- J. Ciccolini: Secretary of the PAMM-EORTC Society.
- J. Ciccolini: Board Member of the European COST Action: CA17140 Nano2Clinics.
- J. Ciccolini: Board Member at the GPCO-Unicancer Society.
- J. Ciccolini: Board Member of the Immuno-Oncology-Unicancer Society.
- J. Ciccolini: Member of the Steering Committee Translationnel of the H&N group at Unicancer.
- J. Ciccolini: Member of the Steering Committee "Anticancer Drugs" at the IATDMCT.
- J. Ciccolini: Member of the Scientific Advisory Board of the School of Pharmacy of Marseille.
- J. Ciccolini: Member of the Scientific Advisory Board of the Prix Fondation ARC Léopold Griffuel.
- F. Gattacceca: president of the groupement des enseignants de pharmacocinétique (GEPK)
- F. Gattacceca: member of the scientific advisory board of the Pharmacy faculty.
- R. Fanciullino: Member of the Steering Committee "Lien Ville-Hôpital-Ville" at the OMEDIT PACA-Corse.
- R. Fanciullino: Coordonator of the Expert Group "Clinical Pharmacy" of the SFPO Society.
10.1.6 Scientific expertise
- S. Benzekry: A*Midex Interdisciplinary call for projects of the Institute for Cancer and Immunology (ICI)
- S. Benzekry: Valorization Committee of the Pharmacy Faculty of Marseille
- J. Ciccolini: Association pour la Recherche sur le Cancer (ARC), CN5 board.
- J. Ciccolini: Institut National du Cancer (INCa), PHRC-K.
- J. Ciccolini: Institut National du Cancer (INCa), CLIPP Innovative Drugs.
- J. Ciccolini: Children's Cancer and Leukaemia Group, Oxford University, England.
10.1.7 Research administration
- A. Rodallec: Website and Social Media coordinator for the CRS benelux Local Chapter
10.2 Teaching - Supervision - Juries
10.2.1 Teaching
- Master S. Benzekry: M Biologie Santé – Parcours IA biomarqueurs (6h).
- DESU S. Benzekry: "Advances courses in pharmacometrics" (10h).
- DESU C. Bigarré: "Advances courses in pharmacometrics" (12h).
- A. Rodallec: lectures in MSc in Pharmacokinetics, MSc in Digipharm, MSc in Innovative Diagnostic and therapeutic Drug Products, DESU "Advances courses in pharmacometrics", DES in animal experiments, Pharm.D. studies (2d, 3d, 4th and 6th year), -> 190 h a year + additional lectures at University of Paris Saclay.
- J. Ciccolini: Founder and Head, Digipharm MSc degree (Aix Marseille Univ). Lectures at Aix Marseille univ in: MSc (2d year) in Oncology, MSc (2d year) in Oncogenetics, MSc (2d year) in Pharmacokinetics, MSc (2d year) in Digipharm, MSc (1st year) in Drugs & Health Products, D.U. in Animal Experiments, D.U. in Genetic Councelling, Master Class in Lung Cancer, Pharm.D. studies (2d, 3d, 4th and 6th year) -> 150 h a year + additional lectures in pharmacokinetics at Université Catholique de Lyon, University of Amsterdam NL and University of Verona Italy.
- R. Fanciullino: Lectures in: MSc (2d year) in Pharmacokinetics, MSc (2d year) in Digipharm, CESU in Oncogeriatry, DES in PK Variability, Pharm.D. studies (3d, 4th and 5th year) -> 190 h a year .
- F. Gattacceca: Director of the Master Program “Pharmacokinetics”.
- F. Gattacceca: Aix-Marseille University school of pharmacy (260h), teaching in other universities (Nîmes, Angers, Montpellier): 90% at a post-graduate level.
- F. Gattacceca: Director of two international University Diplomas: “Modeling and simulation: population approaches in pharmacokinetics/pharmacodynamics” and “Modeling and simulation: physiologically-based pharmacokinetic modeling for pharmacology and toxicology” (the latter created in 2021: first session in January-May 2022).
- F. Gattacceca: 2-days workshop in PBPK, Université de Montréal, Canada, June 2022
- F. Gattacceca: CIVIS Summerschool Drug Design and Discovery, University of Athens, Greece, July 2022
- L. Greillier: M2 Recherche clinique et Simulation en Santé.
- L. Greillier: oncology and pulmonology for 3rd – 11 year medical students.
- X. Muracciole: DIU radio-urology for resident medical student.
- X. Muracciole: DCIU radio surgery for resident medical student
10.2.2 Supervision
- Postdoc: P. Dufossé, 2022 - 2024, QUANTIC, supervision S. Benzekry
- Postdoc: R. Passannante, 2022 - 2023, Thermonano, supervision A. Rodallec
- PhD: L. Nguyen Phuong, 2022 - 2025, SChISM, Mechanistic modeling of circulating DNA combined to machine learning for prediction of response and survival following immunotherapy, supervision S. Benzekry, S. Salas
- PhD: Jessica Ou, 2021-2024, supervision F. Gattacceca, P. Garrigue
- PhD: C. Bigarré, 2020 - 2023, Mathematical modeling for prediction of metastatic relapse in breast cancer, supervision S. Benzekry, X. Muracciole
- PhD: Anthéa Deschamps, 2019-2023, supervision F. Gattacceca, R. Guilhaumou
- PhD: Clémence Marin (APHM, CetuxiMAX), supervision J.Ciccolini.
- PhD: Mathilde Dacos (APHM, Nanimmuno), supervision R.Fanciullino.
- PhD: Guillaume Sicard (APHM, SMART Project), supervision J.Ciccolini, R. Fanciullino.
- PhD: Mourad Hamimed(AMU, Provin & Ovima trials), supervision J.Ciccolini, N.André.
- Engineer: M. Karlsen, 2021 - 2023, QUANTIC, supervision S. Benzekry
- Master 2: Liza Al Shikhley (UTC Compiègne, PIONeeR project), supervision S. Benzekry
- Master 1: Mathieu Lalaque (Centrale Marseille, PIONeeR project), supervision S. Benzekry
- Master 1: Rémy Harle (AMU, METAMATS), supervision S. Benzekry, C. Bigarré
- Master 2: Sophie Marolleau (AMU, Thermonano project), supervision A. Rodallec
- Master 2: Loic Osanno (APHM, Cyta-PGx Project), supervision R. Fanciullino, J.Ciccolini.
- Master 2: Alexis Plan (APHM, MTX Project), supervision J.Ciccolini.
10.2.3 Juries
- J. Ciccolini, Reviewer for the HDR of Julie Bertrand, Université Paris Cité, Dec. 2022
- J. Ciccolini, Reviewer for the HDR of Laurent Bourguignon, Université Lyon 1, Nov. 2022
- J. Ciccolini, Reviewer for the Ph.D of Simon Verdez, Université of Burgondy, Dec. 2022
- J. Ciccolini, Jury Member for the Ph.D of Mathieu Martino, AMU, June 2022.
- J. Ciccolini, Jury Chair for Pharm.D. thesis (Marseille) and Jury Member for PharmD Thesis (Marseille, Montpellier, Lyon, Caen): 15 students /year.
- F. Gattacceca, reviewer for the HDR of Jérôme Henri, ANSES-Université de Rennes 1, Nov 2022
- F. Gattacceca, reviewer for the PhD of Anis Krache, Université Toulouse 3, Feb 2022
- F. Gattacceca, reviewer for the PhD of Thibaud Derippe, Université Paris Cité, Dec 2022
- F. Gattacceca, examiner for the PhD of Sonia Saib, Université de Saint-Etienne Dec 2022
- F. Gattacceca, examiner for the PharmD thesis of Anissa Barakat, Université de Montpellier, Oct 2022
- F. Gattacceca, reviewer for the PharmD thesis of Estelle Pierrot, Université de Montpellier, Sept 2022
- A. Rodallec, reviewer for the Pharm.D. thesis of 3-5 students/year
- X. Muracciole, reviewer for the M.D. thesis of Alice Daumas
- X. Muracciole, Jury Member for the PhD thesis of R. Serre, AMU.
- R. Fanciullino, reviewer for the Pharm.D. thesis of 10 students/year
- R. Fanciullino, reviewer for DES thesis of 10 students/year
10.2.4 Popularization
- J. Ciccolini: Interview for ReseauProSanté: "Activités hospitalo-universitaire en pharmacocinétique" Sep. 2022
- J. Ciccolini: Interview for Observance, Fédération Nationale des Syndicats des Internes en Pharmacie (FNSIP): "Outils Digitaux en sciences pharmaceutiques" Jun. 2022.
- J. Ciccolini: La Lettre du Cancérologue XXX(2): "Le bénéfice du monitoring des Biothérapies en Oncologie" Jan. 2022.
- J. Ciccolini: Rencontres corps enseignant-CRCM, Académie d'Aix Marseille, May 2022.
- J. Ciccolini: Interview VJOncology, Highlights in Pharmacy at ESMO 2022: Personalized Dosing with TKIS. https://www.youtube.com/watch?v=1h7RCN6FOYs
11 Scientific production
11.1 Major publications
- 1 articleComputational oncology — mathematical modelling of drug regimens for precision medicine.Nature Reviews Clinical Oncology134April 2016, 242-254
- 2 articleArtificial Intelligence and Mechanistic Modeling for Clinical Decision Making in Oncology.Clinical Pharmacology and TherapeuticsJune 2020
- 3 articleMathematical modeling of tumor-tumor distant interactions supports a systemic control of tumor growth.Cancer Research2017
- 4 articleModeling Spontaneous Metastasis following Surgery: An In Vivo-In Silico Approach.Cancer Research763February 2016, 535 - 547
- 5 articlePharmacokinetics and Pharmacodynamics-Based Mathematical Modeling Identifies an Optimal Protocol for Metronomic Chemotherapy.Cancer Research77172017, 4723-4733
- 6 articleWomen at a Disadvantage in Fluorouracil Treatment.JAMA oncology26June 2016, 829
- 7 articleA phase Ia/Ib clinical trial of metronomic chemotherapy based on a mathematical model of oral vinorelbine in metastatic non-small cell lung cancer and malignant pleural mesothelioma: rationale and study protocol.BMC Cancer161December 2016, 278
- 8 articleCDA as a predictive marker for life-threatening toxicities in patients with AML treated with cytarabine.Blood Advances25February 2018, 462 - 469
- 9 articleTowards Rational Cancer Therapeutics: Optimizing Dosing, Delivery, Scheduling, and Combinations.Clinical Pharmacology and Therapeutics1083September 2020, 458-470
- 10 articleMachine Learning and Mechanistic Modeling for Prediction of Metastatic Relapse in Early-Stage Breast Cancer.JCO Clinical Cancer Informatics4September 2020, 259-274
11.2 Publications of the year
International journals
International peer-reviewed conferences
Conferences without proceedings
Doctoral dissertations and habilitation theses
Reports & preprints
Other scientific publications
11.3 Other
Scientific popularization
11.4 Cited publications
- 82 articleExploiting Cancer’s Tactics to Make Cancer a Manageable Chronic Disease.Cancers1266Jun 2020, 1649
- 83 articleFrom patient-specific mathematical neuro-oncology to precision medicine..Front Oncol3AprilJan 2013, 62–62
- 84 articleComputational Modelling of Metastasis Development in Renal Cell Carcinoma.PLoS Comput Biol111100007Nov 2015, e1004626
- 85 articleDetermination of the unmetabolized 18F-FDG fraction by using an extension of simplified kinetic analysis method: clinical evaluation in paragangliomas.Medical & biological engineering & computing541Jan 2016, 103–111
- 86 articleOncotarget829Jul 2017, 47161–47166
- 87 articleGlobal Dormancy of Metastases Due to Systemic Inhibition of Angiogenesis.PLoS ONE9100019Jan 2014, e84249-11
- 88 articleMathematical Modeling of Tumor-Tumor Distant Interactions Supports a Systemic Control of Tumor Growth..Cancer Research7718Sep 2017, 5183–5193
- 89 articleModeling Spontaneous Metastasis following Surgery: An In Vivo-In Silico Approach.Cancer Research76300000Feb 2016, 535–547
- 90 articleProgress and Opportunities to Advance Clinical Cancer Therapeutics Using Tumor Dynamic Models..Clinical cancer research : an official journal of the American Association for Cancer ResearchDec 2019, 1–22
- 91 articleStan: A Probabilistic Programming Language.Journal of Statistical Software761Jan 2017, 1–32
- 92 articleEmergence of drug tolerance in cancer cell populations: an evolutionary outcome of selection, nongenetic instability, and stress-induced adaptation.Cancer Research756Mar 2015, 930–939
- 93 articleMechanistic Learning for Combinatorial Strategies With Immuno-oncology Drugs: Can Model-Informed Designs Help Investigators?JCO Precision Oncology4May 2020, 486–491
- 94 articleCombinatorial immunotherapy strategies: most gods throw dice, but fate plays chess.Annals of Oncology3011Nov 2019, 1690–1691
- 95 articleRealistic simulation of the 3-D growth of brain tumors in MR images coupling diffusion with biomechanical deformation.IEEE Transactions on Medical Imaging2410Oct 2005, 1334–1346
- 96 articleA new methodology to derive 3D kinetic parametric FDG PET images based on a mathematical approach integrating an error model of measurement.EJNMMI Research8Nov 2018, URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6238015/
- 97 articlePrediction of the Evolution of Thyroidal Lung Nodules Using a Mathematical Model..82Jan 2010, 37–38
- 98 articleA multi-layered systems approach for renal cell carcinoma.bioRxivJan 2020, 2020.01.13.904235
- 99 articleConvergence of a stochastic approximation version of the EM algorithm.The Annals of Statistics271Zbl: 0932.62094Mar 1999, 94–128
- 100 articleA 2/1 Sunitinib Dosing Schedule Provides Superior Antitumor Effectiveness and Less Toxicity Than a 4/2 Schedule for Metastatic Renal Cell Carcinoma: A Systematic Review and Meta-Analysis.Frontiers in Oncology102020, 313
- 101 articleUsing the SAEM algorithm for mechanistic joint models characterizing the relationship between nonlinear PSA kinetics and survival in prostate cancer patients.Biometrics731Mar 2017, 305–312
- 102 articleNumerical solution of an inverse problem in size-structured population dynamics.Inverse Problems254Apr 2009, 045008–045008
- 103 articleA phase Ia/Ib clinical trial of metronomic chemotherapy based on a mathematical model of oral vinorelbine in metastatic non-small cell lung cancer and malignant pleural mesothelioma: rationale and study protocol.BMC cancer161000002016, 278
- 104 articleIn vitro and in vivo reversal of resistance to 5-fluorouracil in colorectal cancer cells with a novel stealth double-liposomal formulation.British Journal of Cancer977Oct 2007, 919–926
- 105 articleMethodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids.Life Sciences254Aug 2020, 117784
- 106 articlePembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung.Cancer. N Engl J Med3782078–20922018
- 107 articleThe evolutionary history of lethal metastatic prostate cancer.Nature5207547Apr 2015, 353–357
- 108 articleThe elements of statistical learning: data mining, inference, and prediction, Springer Series in Statistics.2009
- 109 articleRevisiting dosing regimen using PK/PD modeling: the MODEL1 phase I/II trial of docetaxel plus epirubicin in metastatic breast cancer patients.Breast Cancer Research and Treatment1562Mar 2016, 331–341
- 110 articleDevelopment of ipilimumab: contribution to a new paradigm for cancer immunotherapy.Seminars in Oncology375Oct 2010, 533–546
- 111 articleCalibrating a Predictive Model of Tumor Growth and Angiogenesis with Quantitative MRI.Annals of Biomedical Engineering477Jul 2019, 1539–1551
- 112 articleSingle-agent paclitaxel for the treatment of breast cancer: an overview.Seminars in Oncology231 Suppl 1Feb 1996, 4–9
- 113 articleQuantitative evidence for early metastatic seeding in colorectal cancer.Nature Genetics5177Jul 2019, 1113–1122
- 114 articleRevisiting Bevacizumab + Cytotoxics Scheduling Using Mathematical Modeling: Proof of Concept Study in Experimental Non-Small Cell Lung Carcinoma..CPT: Pharmacometrics & Systems Pharmacology71Jan 2018, 42–50
- 115 articleA Dynamical Model for the Growth and Size Distribution of Multiple Metastatic Tumors.Journal of Theoretical Biology2032Mar 2000, 177–186
- 116 articleDynamic Risk Profiling Using Serial Tumor Biomarkers for Personalized Outcome Prediction.Cell1783Jul 2019, 699-713.e19
- 117 bookMixed Effects Models for the Population Approach.CRC PressJul 2014
- 118 articleNonlinear mixed effects models for repeated measures data..Biometrics463Sep 1990, 673–687
- 119 articleA phase II randomized trial of nivolumab with stereotactic body radiotherapy (SBRT) versus nivolumab alone in metastatic (M1) head and neck squamous cell carcinoma (HNSCC)..Journal of Clinical Oncology3615_supplMay 2018, 6009–6009
- 120 articleRevisiting Dosing Regimen Using Pharmacokinetic/Pharmacodynamic Mathematical Modeling: Densification and Intensification of Combination Cancer Therapy.Clinical Pharmacokinetics55800000Aug 2016, 1015–1025
- 121 articleModel driven optimization of antiangiogenics + cytotoxics combination: application to breast cancer mice treated with bevacizumab + paclitaxel doublet leads to reduced tumor growth and fewer metastasis.Oncotarget814Feb 2017, 23087–23098
- 122 articleIn Vivo Bioluminescence Tomography for Monitoring Breast Tumor Growth and Metastatic Spreading: Comparative Study and Mathematical Modeling.Scientific Reports600000Nov 2016, 36173
- 123 articleIntegrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas.Nature Biotechnology3833Mar 2020, 333–342
- 124 articleAvelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma.New England Journal of Medicine38012Mar 2019, 1103–1115
- 125 phdthesisMathematical modelling of neoadjuvant antiangiogenic therapy and prediction of post-surgical metastatic relapse in breast cancer patients.Université de BordeauxOct 2019, URL: https://tel.archives-ouvertes.fr/tel-02560543
- 126 articleMatrix metalloproteinases at cancer tumor–host interface.Seminars in Cell & Developmental Biology191Feb 2008, 52–60
- 127 articleAn alternative parameter for early forecasting clinical response in NSCLC patients during radiotherapy: proof of concept study.The British Journal of Radiology891062Jun 2016, 20160061
- 128 articleFrom 3D spheroids to tumor bearing mice: efficacy and distribution studies of trastuzumab-docetaxel immunoliposome in breast cancer.International Journal of Nanomedicine132018, 6677–6688
- 129 articleDefining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies.Annals of Oncology: Official Journal of the European Society for Medical Oncology2772016, 1190–1198
- 130 articleOptimal Scheduling of Bevacizumab and Pemetrexed/cisplatin Dosing in Non-Small Cell Lung Cancer.CPT: pharmacometrics & systems pharmacologyApr 2019
- 131 articleAtezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC..New England Journal of Medicine37824Jun 2018, 2288–2301
- 132 articleComprehensive analysis of the clinical immuno-oncology landscape..Ann. Oncol.291Jan 2018, 84–91
- 133 phdthesisMathematical modeling of antibody nanoconjugates transport in tumors.Bordeaux UniversityDec 2020
- 134 articlePREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer..Breast cancer research121Jan 2010, R1

