Section: Research Program
Excitable systems: analysis of physiological time series
The research described in this section is a collaboration effort of GEOSTAT, CNRS LOMA (Laboratoire Ondes et Matière d'Aquitaine) and Laboratory of Physical Foundation of Strength, Institute of Continuous Media Mechanics (Perm, Russia Federation).
Presentation and objectives
Provide state of the art, cutting-edge tools to intra-cardiac multiscale analysis of the electrical activity of fibrillating hearts ; offer physiological hypotheses likely to account for the new quantitative observations together with quantitative simulations.
Results
Wavelet-based methods (WTMM, log-cumulants, two point scale correlations), and confidence statistical methodology, have been applied to catheter recordings in the coronary sinus vein right next to the left atria of a small sample of patients with various conditions, and exhibit clear multifractal scaling without cross-scale correlation, which are coined “multifractal white noise,” and that can be grouped according to two anatomical regions. We show that this is incompatible with the common lore for atrial fibrillation based on so-called circuit reentries. In a new description, we propose that circuit reentries may well exist before the onset of fibrillation, favoring onset but not contributing directly to the onset and perpetuation. By contrast, cell-to-cell coupling is considered fundamentally dynamical. The rationale stems from the observation that multifractal scaling necessitates a high number of degrees of freedom (tending to infinity with system size), which can originate in excitable systems in hyperbolic spatial coupling. In other words, common mathematical models for fibrillation which insist on the intrinsic chaotic dynamics of excitable cells coupled by elliptic propagators (like diffusion) are immune to multifractal scaling. Within this framework, we have developed a new hypothesis in physiology, backed by a mathematical model of gap junction conductance kinetics, that is capable of yielding correct spectra, all based on otherwise known physiology.
Interpretation
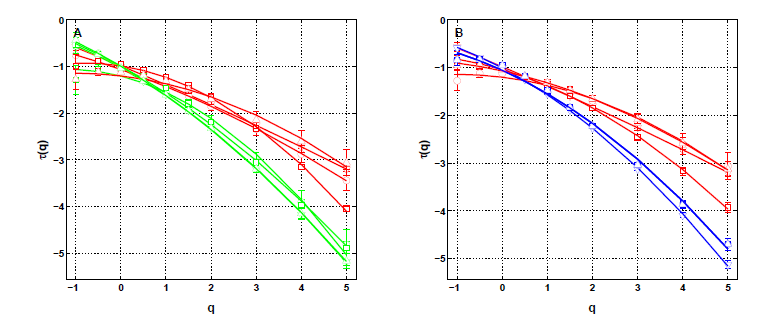
Atrial Fibrillation (AF) is an arrhythmia originating in the rapid and irregular electrical activity of the atria (the heart’s two upper chambers) that causes their pump function to fail, increasing up to fivefold the risk of embolic stroke. The rate of AF recurrences after an initial ablation procedure treating paroxysmal AF increases with time, necessitating multiple redos, and most patients suffering persistent AF are resistant to treatment. The prevailing electrophysiological concepts describing tachy-arrhythmias are more than a century old. They involve abnormal automaticity and conduction. Initiation and maintenance are thought to arise from a vulnerable substrate prone to the emergence of multiple self-perpetuating reentry circuits, also called “multiple wavelets”. We have analyzed the complexity of voltage signals recovered with bipolar electrodes in the CS during AF. We used two declinations of a wavelet-based multi-scale method, the moment (partition function) method and the magnitude cumulant method, as originally introduced in the field of fully developed turbulence. In the context of cardiac physiology, this methodology was shown to be valuable in assessing congestive heart failure from the monitoring of sinus heart rate variability (Ivanov, et al. Nature 399, 461–465) [42]. We develop a model such that the substrate function is modulated by the kinetics of conduction. A simple reversible mechanism of short term remodeling under rapid pacing is demonstrated, by which ionic overload acts locally (dynamical feedback) on the kinetics of gap junction conductance. The whole process may propagate and pervade the myocardium via electronic currents, becoming desynchronized. Contrary to existing mathematical models based on circuit reentries, a spatio-temporal multifractal intermittent dynamics emerges similar to the one found in the CS, opening a new avenue towards the understanding of AF mechanisms of perpetuation. We have shown that the wavelet-based multifractal analysis of long time series of the local impulse energy recorded in the CS of a patient with chronic AF was able to reveal and quantify the intermittent nature of these signals at low frequency (f < 2 Hz). To our knowledge, this research is the first to report on the observation and quantification of such multifractal dynamics of the endocavitary electrical activity during AF which is found more complex than previously suspected. Two main observations can be made: (i) the local impulse energy displays different multifractal properties in the left atrial wall area than in the ligament of Marshall area consistently with different anatomical substrate conditions, and (ii) while recorded along the CS vein, the local impulse energy does not exhibit long-range dependence associated with an underlying multiplicative cascade, or in other words the multifractal distribution of the singularities inferred by the two point magnitude analysis does not display any correlation across scales just like a log-normal “multifractal white noise”.
This analysis definitely challenges current knowledge in physical, physiological and clinical fundamentals of AF arrhythmia.
The absence of an underlying cascading process is not such a surprise since underlying the multifractal properties displayed by the local impulse energy at low frequencies ( Hz), there is no clear 3D “fragmentation” process inducing some cascading of energy from large to small time scales and also no obvious 2D “aggregation, coalescence or growth” process bringing energy from small to large time scales. What are the physical and physiological mechanisms that drive the multifractal nature of local impulse energy and give rise to the observed differences according to area is still an open question. Nonetheless, these results already undermine the commonly accepted concepts revolving around circuit reentries, and a fortiori spiral waves, as being basic mechanisms for the onset and perpetuation of AF. The mechanistic “wavelength” criterion indeed conveys the idea that random spatio-temporal dispersion of refractoriness, or more generally of functional properties, leads to random mixing of circuit reentries. The “wavelength” scale adjusts naturally to the typical scale of dispersion when it exists as would be the case for Gaussian statistics of dispersion. In that case, the statistics of the local impulse energy remains Gaussian throughout scales. On the contrary, to fit our new observations we see that the statistics is not Gaussian and evolves across scales through a log-normal propagation law, which accounts for the intermittency, over the range of a few beat cycles ( s) to several tens ( s and possibly more), therefore spanning the whole atria. Although the ligament of Marshall area is highly innervated, it is quite unlikely that modulations by the ANS, that affects primarily heart rate, play a significant role in the intermittent dynamics, since the documented three peak frequencies at 0,4 Hz, 0,15 Hz and 0,04 Hz do not show up in our analysis. Furthermore, we have found at least two areas with different multifractal regimes. See figure 1.
Therefore, our findings raise new challenging questions calling for ongoing efforts to develop physiological heart tissue models that account for the low frequency intermittent nature of local impulse energy. In this spirit, in an ongoing research, we propose a model of gap junction conduction remodeling in a denervated heart that accounts for the observed intermittent dynamics over large time scales, as resulting from incoherent random back scatterings, leading to the desynchronization of the network of cardiac excitable cells.
These results have been accepted in a Frontiers in Physiology paper to be published in 2018 https://hal.inria.fr/hal-01673364.
|