Section: New Results
Human vision understanding through joint experimental and modeling studies, for normal and distrophic vision
From micro- to macroscopic description of the retina
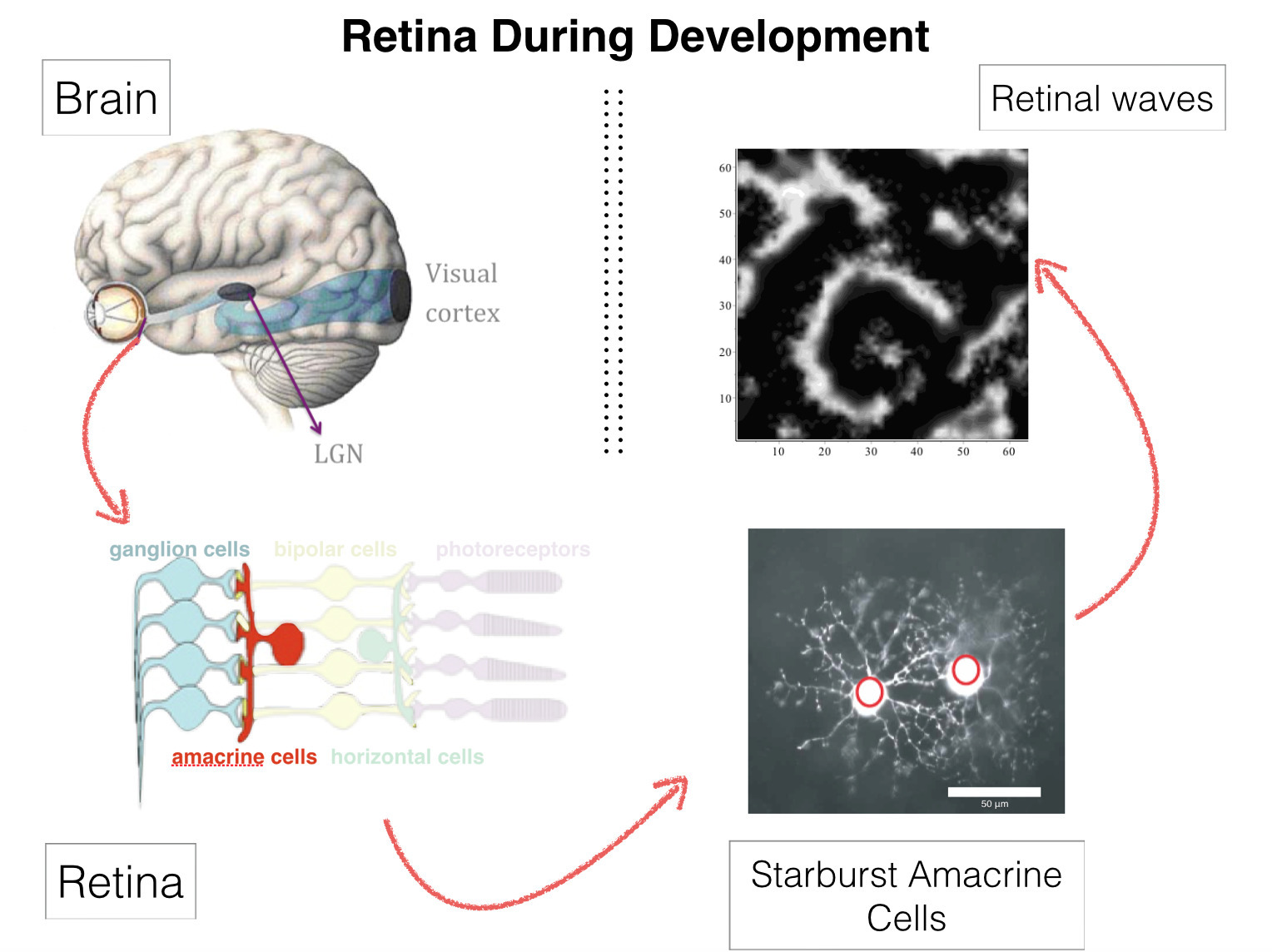
Retinal Waves
Participants : Dora Matzakos-Karvouniari [Laboratoire Jean-Alexandre Dieudonné, (LJAD), Nice, France] , Bruno Cessac, Lionel Gil [Institut de Physique de Nice (InPhyNi), France] .
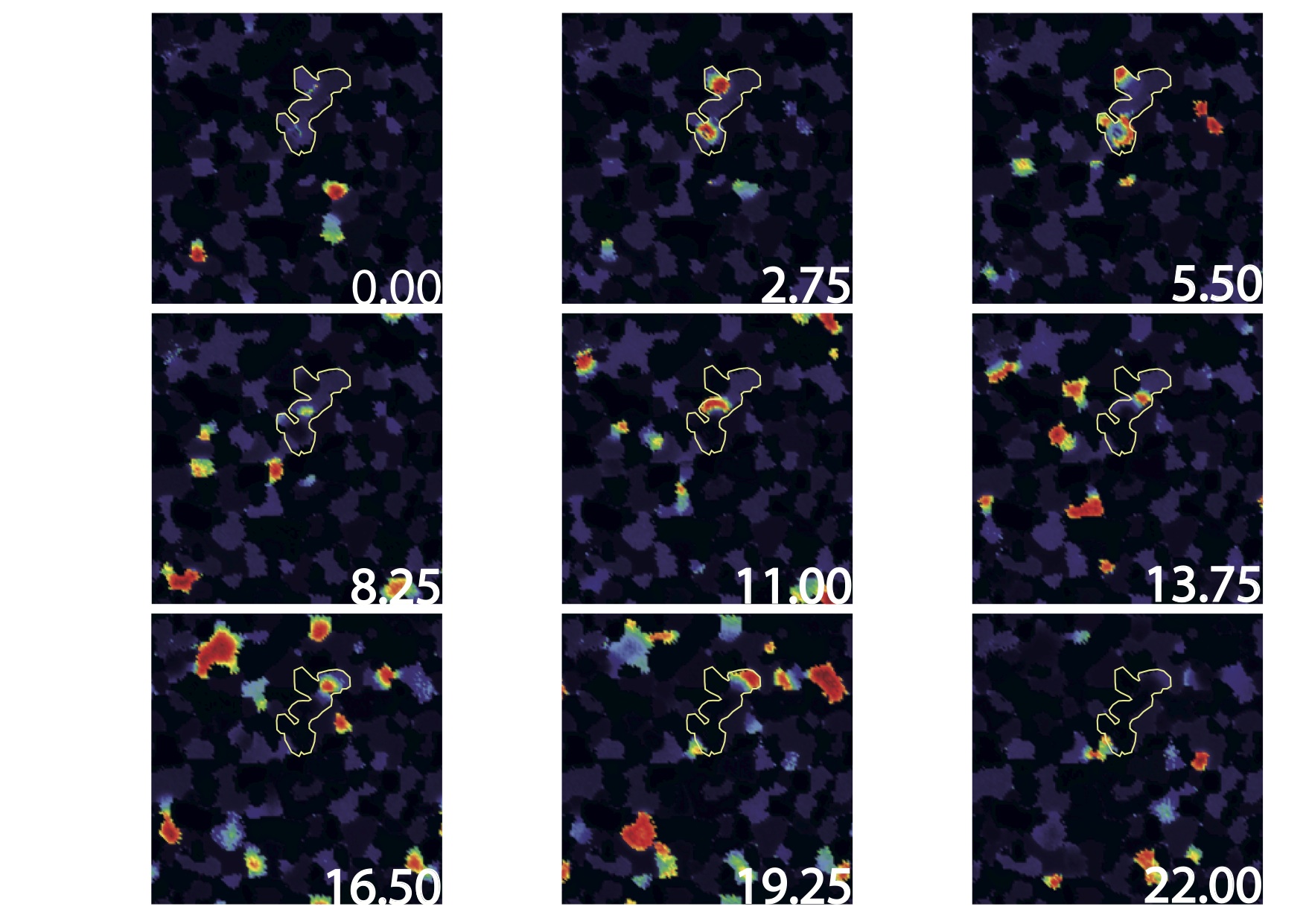
Retinal waves are bursts of activity occurring spontaneously in the developing retina of vertebrate species, contributing to the shaping of the visual system organization: retina circuitry shaping, retinotopy, eye segregation [63], [47], [58], [48]. They stop a few weeks after birth. Wave activity begins in the early development, long before the retina is responsive to light. It was recently found that they can be reinitiated pharmacologically in the adult mammalian retina [46]. This could have deep consequences on therapy for several degenerative retinal diseases. The mechanism of their generation, in developing, or adult retinas, remains however incompletely understood [64].
We have proposed a model for stage II retinal waves - induced by bursting Starburst Amacrine Cells (SACs) coupled by acetylcholine - with two objectives: (i) being sufficiently close to biophysics to explain and propose experiments and (ii) affording a mathematical analysis [14], [34]. From a bifurcations analysis we have highlighted several relevant biophysical parameters controlling waves generation, mainly regulating potassium and calcium dynamics. We thus explain how SACs in different species exhibit a large variability in their bursting periods with a common mechanism.
|
Based on this biophysical model we have analysed here the dynamics of retinal waves and their statistics. We show that, despite the acetylcholine coupling intensity has been experimentally observed to change during development, SACs retinal waves can nevertheless stay in a regime with power law distributions, reminiscent of a critical regime. Thus, this regime occurs on a range of coupling parameters instead of a single point as in usual phase transitions. We explain this phenomenon thanks to a coherence-resonance mechanism, where noise is responsible for the broadening of the critical coupling strength range. This work has been presented in [14], [16], [25]
|
Anticipation in the retina and the visual cortex V1
Participants : Bruno Cessac, Frédéric Chavane [Institut de Neurosciences de la Timone (CNRS and Aix-Marseille Université, France)] , Alain Destexhe [Institute de Neuroscience Paris-Saclay (UNIC)] , Sandrine Chemla [Institut de Neurosciences de la Timone (CNRS and Aix-Marseille Université, France)] , Selma Souihel, Matteo Di Volo [Institute de Neuroscience Paris-Saclay (UNIC)] .
This work has been done in the context of the ANR Trajectory and Selma Souihel Thesis [11].
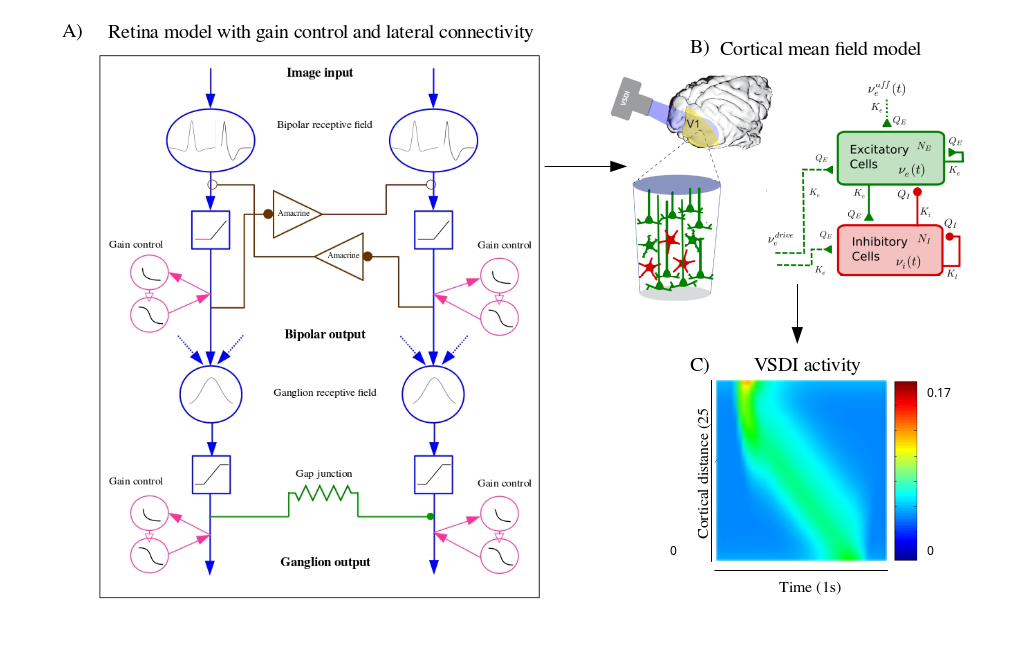
Vision is initiated in the retina, where light is converted into electrical signals by photoreceptors, sent to bipolar cells then ganglion cells, generating spike trains. Visual information is then transmitted to the thalamus via the optic nerve which in turn transmits it to the visual cortex. The retinal processing alone takes time, up to 150 ms, not to mention the time lags introduced by synaptic transmissions between the three processing units. This shows that the existence of compensatory mechanisms to reduce processing delays is absolutely essential. These compensatory mechanisms are known as anticipation. Anticipation first occurs at the level of the retina and is further carried out by the primary visual cortex. In its first occurrence, anticipation is either characterized by a shift in the the peak response, or a short range wave of activation. In the second case, it is characterized by a wider range wave of activation.
The first contribution of this work is the development of a generalized 2D model of the retina, mimicking three types of ganglion cells : Fast OFF cells with gain control, direction selective cells with gap junction connectivity, and differential motion cells connected through an upstream amacrine circuit, able of anticipating different kinds of moving stimuli. The second contribution is to use our retina model as an input to a mean field cortical model to reproduce motion anticipation as observed in voltage sensitive dye imaging recordings. Throughout our work, we will study the effect of non linear phenomena involved in anticipation, as well as connectivity, both at the level of the retina and the primary visual cortex. The integrated retino-cortical model allowed us to study the effects of anticipation on two-dimensional stimuli, and to highlight the collaborative aspect of anticipation mechanisms in the retina and the cortex.
|
This work has been presented in [17], [22], [20], [34], [21], [11]
Dynamical synapse in the retina
Participants : Bruno Cessac, Simone Ebert, Olivier Marre [Institut de la Vision (IdV), Paris, France] , Romain Veltz [MathNeuro] .
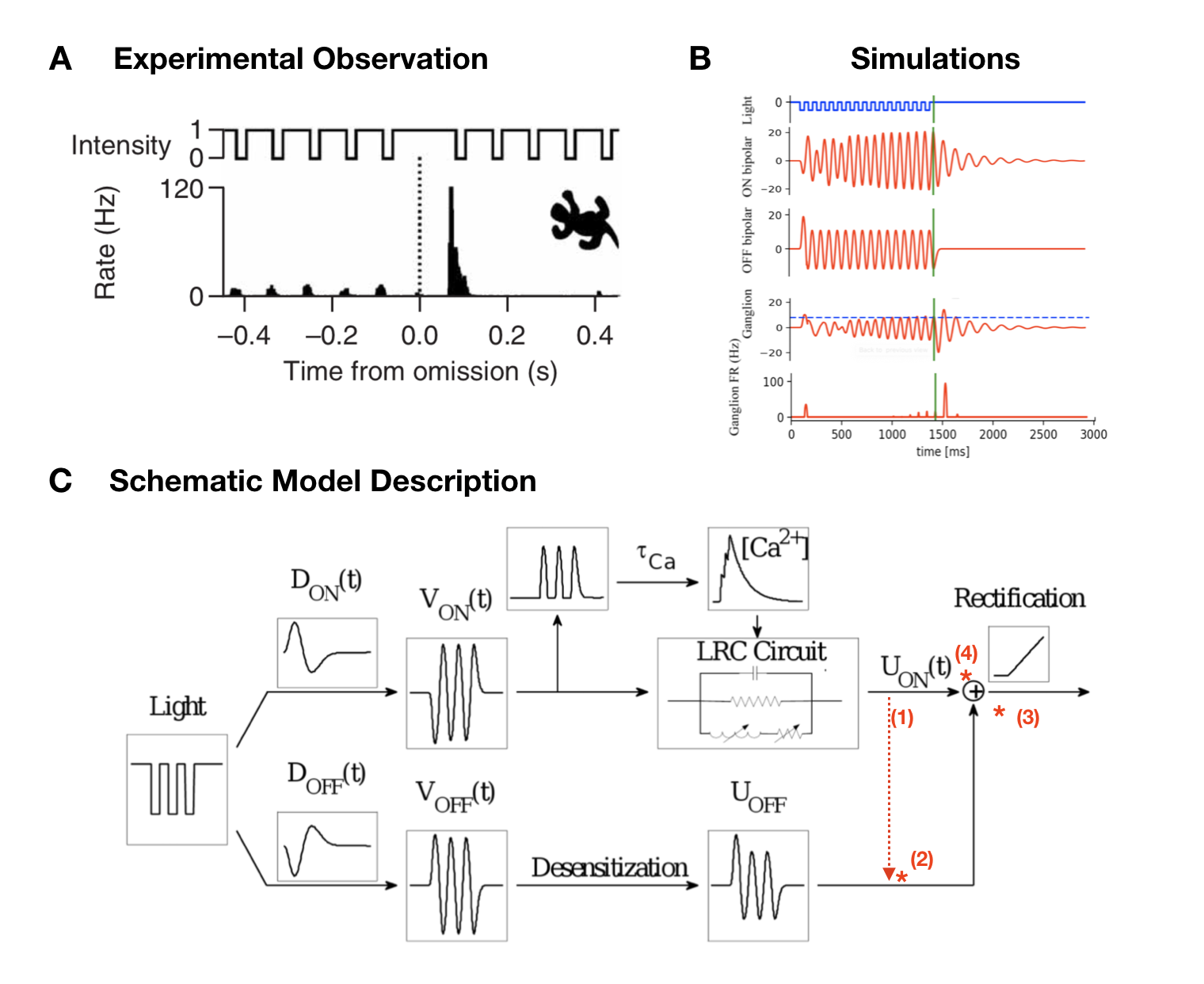
A very sophisticated example of the computations within the retina is observed when the visual system is exposed to a periodic stimulus, such as a regular series of flashes. If the retina would simply respond proportionally to the stimulus input, one might expect that ganglion cells would become entrained into aperiodic activity, responding to each flash. When the stimulus sequence ends,the activity would end as well. However, ganglion cells can exhibit various different kinds of response patterns to this form of stimulation shown in Figure 3. At the beginning of the stimulus, cells typically respond to the first flash of this new stimulus with a peak of activity, but then very rapidly decay in the amplitude of their response to the following flashes. Most remarkably, when the flash sequence ends ganglion cells do not just stop to respond, but in fact may generate a pulse of activity signalling the missing stimulus. This property of indicating a deviation from an expected pattern has been termed the Omitted Stimulus Response (OSR) (Schwartz, Harris, Shrom, Berry, 2007). The aim of this study was to implement and compare the two existing models of Omitted Stimulus Response in the retina, as well as exploring potential mechanisms that may be involved in generating it. Especially synaptic mechanisms may provide an explanation here, but the integration of such a mechanism into an OSR model has not been explored yet. A potential synaptic property that provides an interesting candidate to test here would be short-term plasticity (STP), which modulates synaptic efficacy depending on the previous activity in a short time interval (Blitz, Foster, Regehr, 2004). STP thus modulates signal transmission and a consecutive spike pattern and has been found to take place within the retina (Dunn, Rieke, 2008). Examining the models’ underlying mechanisms, advantages and disadvantages as well as similarities and differences will provide a good foundation to modify existing models by adding potential mechanisms and exploring their effect on a ganglion cell response to a periodic stimulus. Ultimately, this may help shedding light on cellular properties in a neuronal circuit as of as few as 3 cells can contribute to already interpreting information from the environment.
This work has been presented in [32]. It has lead to experiments done in the Institut de la Vision by S. Ebert (internship Biovision) and O. Marre (Institut de la Vision (IdV), Paris, France) in November 2019.
|
Numerical modelling of the retina in normal and pathological conditions
Probing retinal function with a multi-layered simulator
Participants : Bruno Cessac, Gerrit Hilgen [Institute of Neuroscience (ION), Newcastle, UK] , Evgenia Kartsaki, Evelyne Sernagor [Institute of Neuroscience (ION), Newcastle, UK] .
Our brain can recreate images from interpreting a stream of information emitted by one million parallel channels in the retina. This ability is partly due to the astonishing functional and anatomical diversity of the retinal ganglion cells (RGCs), each interpreting a different feature of the visual scene. How precisely this complexity is encoded in the spike trains produced by the population of RGCs is, however, largely unknown. Adding to the complexity, RGCs “speak” to each other during complex tasks (especially motion handling), via amacrine cells (ACs - lateral connectivity). To decipher their role, we study an experimental setting where neurons co-express the genes Grik4 or Scnn1a and excitatory or inhibitory DREADDs (De-signer Receptors Exclusively Activated by Designer Drugs), activated by the designer drug CNO. Switching on or off RGCs and/or ACs cells may not only impact the RGCs individual response but also their concerted activity to different stimuli, thus allowing us to understand how they contribute to the encoding of complex visual scenes. However, it is difficult to distinguish on pure experimental grounds the effect of CNO when both cell types express DREADDs, as these cells “antagonise” each other. Contrarily, numerical simulation can afford it. Here, we propose a novel simulation platform that can reflect normal and impaired retinal function (from single-cell to large-scale level). It is able to handle different visual processing circuits and allows us to visualise responses to visual scenes (movies). In addition, the platform allows simulation of retinal responses when DREADD-expressing cell subclasses are either silenced or excited with CNO. To demonstrate how our simulator works, we deploy a circuit that handles motion on a large-scale level and study how the retina responds to visual scenes by visualising retinal processing at each level. The simulator also provides a tunable parameter to control the CNO effect (excitation or inhibition). Consequently, it facilitates the disentanglement of the effect of CNO on ACs and RGCs. Nevertheless, simulations and experiments are widely complementary. Experiments are necessary to constrain the numerical model and check its validity (especially, its predictions), while the computational approach affords to explore aspects that cannot be easily achieved experimentally.
This work has been presented in [24], [19], [33]
Simulating the cortical activity evoked by artificial retinal implants
Participants : Teva Andréoletti, Bruno Cessac, Frédéric Chavane [Institut de Neurosciences de la Timone (CNRS and Aix-Marseille Université, France)] , Sébastien Roux [Institut de Neurosciences de la Timone (CNRS and Aix-Marseille Université, France)] .
Recent advances in neuroscience and microelectronics opens up the possibility of partially restoring vision to blind patients using retinal prostheses. These are devices capturing the light of a visual scene and converting it to electric impulses sent by a matrix of electrodes chirurgically fixed on the retina. The simulation of an electrode elicits an activation in the visual cortex that evokes a percept similar to a light spot called phosphene. The joint stimulation of electrodes allows to reproduce simple shapes (letters, objects, stairs) and to restore a low resolution vision to blind people (see Fig 1). This domain of research is however at an early stage compared to cochlear implants. Especially, the way an electric stimulation activates the visual cortex is still poorly understood. The group of F. Chavane (NeOpTo team at INT Marseille) has used mesoscopic recordings of cortical activity (optical imaging) to better understand the activity evoked by stimulation of the retina with implanted multi electrodes arrays (Roux et al 2016 eLife). Their results show that local stimulation of the retina evoked a cortical activity that is up to 10 times larger than what is expected based on the activity evoked by visual stimuli. This result is in line with known poor resolutions of percepts evoked by stimulation of artificial retinas implanted in blind patients. This observed spread of evoked cortical activity is now better understood. An important effect, evidenced by Roux et al (2017) https://elifesciences.org/articles/12687 is the asymmetrical spread of electric activity induced by the direct activation of retinal cells axons away from their somata.
This effect can be modelled at the level of a single electrode with a significant match to experimental measurement. Retinal prostheses integrate hundreds of electrodes and this model can be used to anticipate the simultaneous activation of several electrodes reproducing the shape of an object (Fig 1). This figure has been produced by a retina simulator, called Macular, developed by the Biovision team at Inria, and aiming at reproducing the retina response to stimulation in normal (stimulation by light) and pathological conditions (electric stimulation by prostheses) https://team.inria.fr/biovision/macular-software/ . In a previous work https://hal.inria.fr/hal-02292831 [28], [29] we have been able to numerically model the effect of the static joint stimulation of electrodes in retina prostheses on the primary visual cortex (V1) and to compare it to normal vision.
|