Section: New Results
Modelling of the liver
Participants : François Bertaux [Imperial College, London] , Noémie Boissier, Dirk Drasdo, Géraldine Cellière, Adrian Friebel, Group Heinzle [Univ. Saarbruecken, Germany] , Group Hengstler [IfADo, Germany] , Stefan Hoehme, Tim Johann, Irène Reo [Vignon-Clementel] , Paul Van Liedekerke, Eric Vibert [Hopital Paul Brousse] , Group Zerial [Max-Planck Inst. for Molecular Genetics, Dresden, Germany] , Groups Iflow, Notox, Vln.
Ammonia detoxification after drug-induced damage
Overdosing acetaminophen (APAP) is the main reason for acute liver failure in the US and UK. Overdose of APAP destroys the hepatocytes localised in the center of each liver lobule (pericentral damage), the repetitive functional and anatomical tissue units of liver. The Human has about 1 million of such lobules. As a consequence, the blood is not sufficiently detoxified from ammonia, which is toxic to the body and can lead to encephalopathy. In France about 1000 cases occur with ammonia toxicity each year. In recent papers we demonstrated by an integrated model that the widely accepted scheme of key reactions for ammonia detoxification is insufficient to explain ammonia detoxification after pericentral lobule damage and predicts a missing ammonia sink [71]. The integrated model couples ODEs representing the consensus reactions in the spatial temporal liver lobule regeneration model. This finding has triggered new experiments leading to the identification of a widely ignored but fundamentally important ammonia sink mechanism. We could show by testing a number of different mechanisms within novel models that this sink mechanism was the only one able to explain the data [74] (and Geraldine Cellière's PhD thesis [3], 2016). The reaction turned out to have the potential to be used in therapeutics by injection of a molecular cocktail triggering it.
In a follow-up work, the ammonia detoxifying reactions have been integrated into each hepatocyte of the previously established tissue-level liver lobule model of regeneration. The final multi-level model simulates blood flow, transport of metabolites and detoxification of ammonia in every hepatocyte of a regenerating lobule. This multi-level model could validate the missing ammonia sink found in the integrated model in ref. [74] but yields differences to the integrated model if the ammonia sink mechanism is integrated. Still by reparameterisation, adding the ammonia sink mechanism, the model is able to explain the data but the results clearly show that spatio-temporal modelling can give results different from pure compartment modelling. In the case of quantitative modelling in pharmacology or toxicology this can be fundamental. We were able to analyse and generalise these findings.
Predicting in vivo drug toxicity from in vitro data
In vitro experiments on APAP (aka paracetamol, acetaminophen) have been used to calibrate a model of APAP drug toxicity using in vitro data, modifying this model to predict in vivo toxicity. This procedure is aimed at as a general pathway from cosmetic and pharmaceutical companies to eliminate or at least reduce animal experiments and, in perspective, permit a better prediction of drug toxicity in the Human. Three critical differences between in vitro and in vivo were stepwise integrated in the model calibrated with in vitro toxicity data to study their impact on in vivo toxicity predictions. (1) The temporal drug exposure profile, (2) the temporal concentration profile of a class of key enzymes, CYP enzymes. Only in hepatocytes in which CYP enzymes are present is APAP metabolised and can downstream cell death occur. (3) The liver architecture represents critical differences in the spatial distribution of the drug. The results are in preparation for publication (Géraldine Cellière's PhD thesis 2016, Cellière et. al., in preparation).
Liver cancer
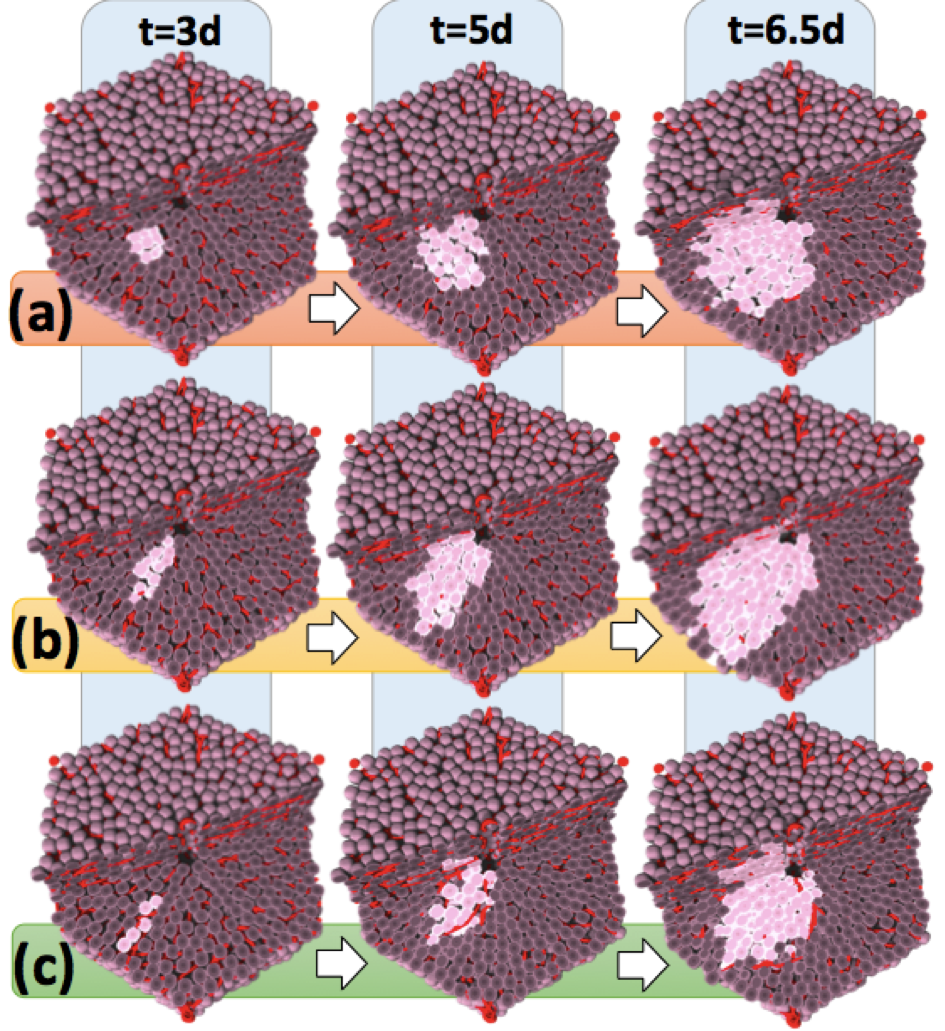
The aggressiveness of a tumour may be reflected by its micro-architecture. To gain a deeper understanding of the mechanisms controlling the spatial organisation of tumors at early stages after tumour initiation, we used an agent-based spatio-temporal model previously established to simulate features of liver regeneration [76]. This model was further developed to simulate scenarios in early tumour development, when individual initiated hepatocytes gain increased proliferation capacity [37]. The model simulations were performed in realistic liver microarchitectures obtained from 3D reconstruction of confocal laser scanning micrographs. Interestingly, the here established model predicted that initially initiated hepatocytes arrange in elongated patterns. Only when the tumour progresses to cell numbers of approximately does it adopt spherical structures. This model prediction was validated by the analysis of initiated cells in a rat liver tumour initiation study using single doses of 250 mg/kg of the genotoxic carcinogen N-nitrosomorpholine (NNM). Indeed, small clusters of GST-P positive cells induced by NNM were elongated, almost columnar, while larger GDT-P positive foci of approximately the size of liver lobules, adopted spherical shapes. Simulation of numerous possible mechanisms demonstrated that only hepatocyte-sinusoidal-alignment (HSA), a previously discovered order mechanism involved in the coordination of liver tissue architecture, could explain the experimentally observed initial deviation from spherical shape. The present study demonstrates that the architecture of small hepatocellular tumour cell clusters early after initiation is still controlled by physiological control mechanisms. However, this coordinating influence is lost when the tumour grows to approximately cells, leading to further growth in spherical shape (Figure 4). Our findings stress the potential importance of organ micro-architecture in understanding tumour phenotypes.
|