Section: Overall Objectives
Presentation
Dracula is a joint research team between Inria, University of Lyon 1 (UCBL) and CNRS (ICJ, UMR 5208 and CGMC UMR 5534). It was created in January 2011.
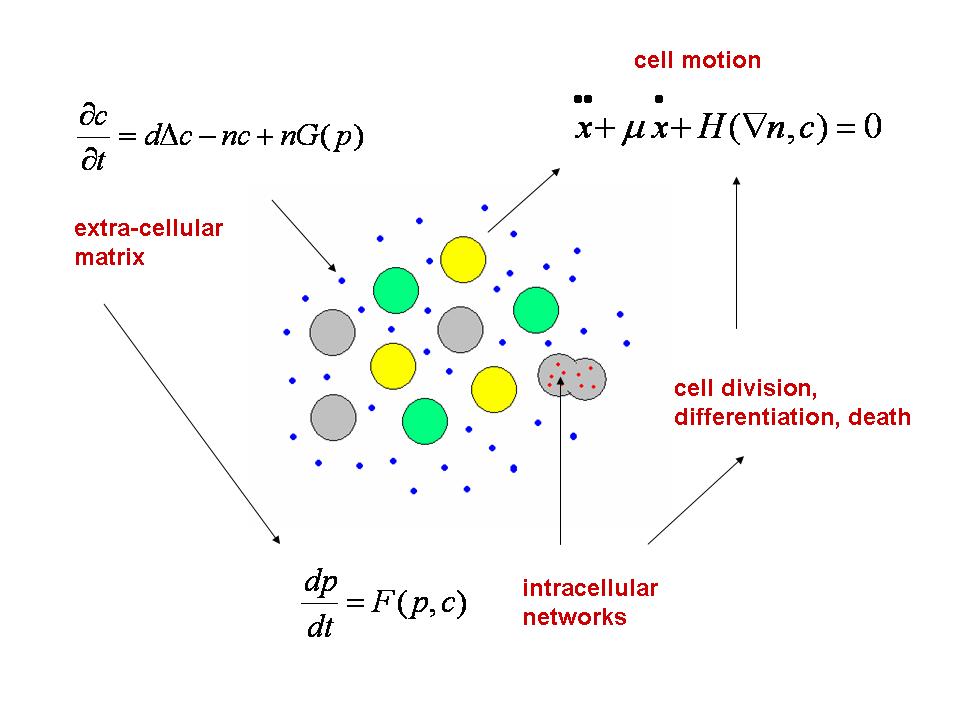
The Dracula project is devoted to multi-scale modeling in biology with applications to normal and pathological hematopoiesis (blood cell production). Multi-scale modeling implies simultaneous modeling of intra-cellular networks (molecular level), of cell behavior (cellular level), of the dynamics of cell populations (organ or tissue) with the control by other organs (organism) (see Figure 1 ). Such modeling represents one of the major challenges in modern science due to its importance and because of the complexity of biological phenomena and of the presence of very different scales.
Hematopoiesis is a complex process that begins with primitive hematopoietic stem cells and results in formation of mature cells: red blood cells, white cells and platelets. Blood cells are produced in the bone marrow, from where mature cells are released into the blood stream. Hematopoiesis is based on a balance between cell proliferation (including self-renewal), differentiation and apoptosis (programmed cell death). The choice between these three possibilities is determined by intra-cellular regulatory networks and by numerous control mechanisms in the bone marrow (see Figure 2 ) or carried out by other organs. Intra-cellular regulatory networks are complex biochemical reactions involving proteins, enzymes and signalling molecules. Thus, hematopoiesis is a complex process which has a vital importance for the organism. Its malfunctioning can result in numerous blood diseases including leukemia.
|
Multi-scale modeling in hematopoiesis holds a great potential. A variety of techniques exists to deal with this problem. However, the complexity of the system poses new difficulties and leads to the development of new tools. The expected results of this study are numerous. On one hand, it will shed new light on the different physiological mechanisms that converge toward the continuous regeneration of blood cells, for example: the behavior of hematopoietic stem cells under stress conditions, the understanding of deregulation of erythropoiesis (the process of red blood cell production) under drag treatments (this can lead to lack of red blood cells (anemia), or a surplus of red blood cells), the understanding of immune response process under the control of T-cell activation and memory cell generation, in order to adapt infection prevention strategies.
On the other hand, the modeling methods developed here for hematopoiesis are relevant to study other complex biological systems. We pay a special attention on developing methods that are not restricted to hematopoiesis. In parallel with hematopoiesis modeling, most of members of Dracula keep on working on modeling of other biological phenomena, for example: tumor cells, prion disease, adaptive dynamics, atherosclerosis, and so on. Approaches developed in the present project are very likely relevant in these fields too.
An important part of our researches is in close collaboration with biologists and physicians in order to stay in close contact with the biological and medical goals. The presence, within the project, of a biologist (Olivier Gandrillon) that has acquired over the years the know-how required for interacting with mathematicians is probably one of the main asset of the project. He participates actively in many tasks of our program, especially involving description of biological process, and he is "consultant" for other biological aspects, in the other parts of the project.