Section: Research Program
Modeling of complex anatomical environments
Objectives:
Milestones:
-
Integration of hyper-elastic materials and higher-order elements
-
Combined behaviors (e.g. electro-mechanical model of the heart)
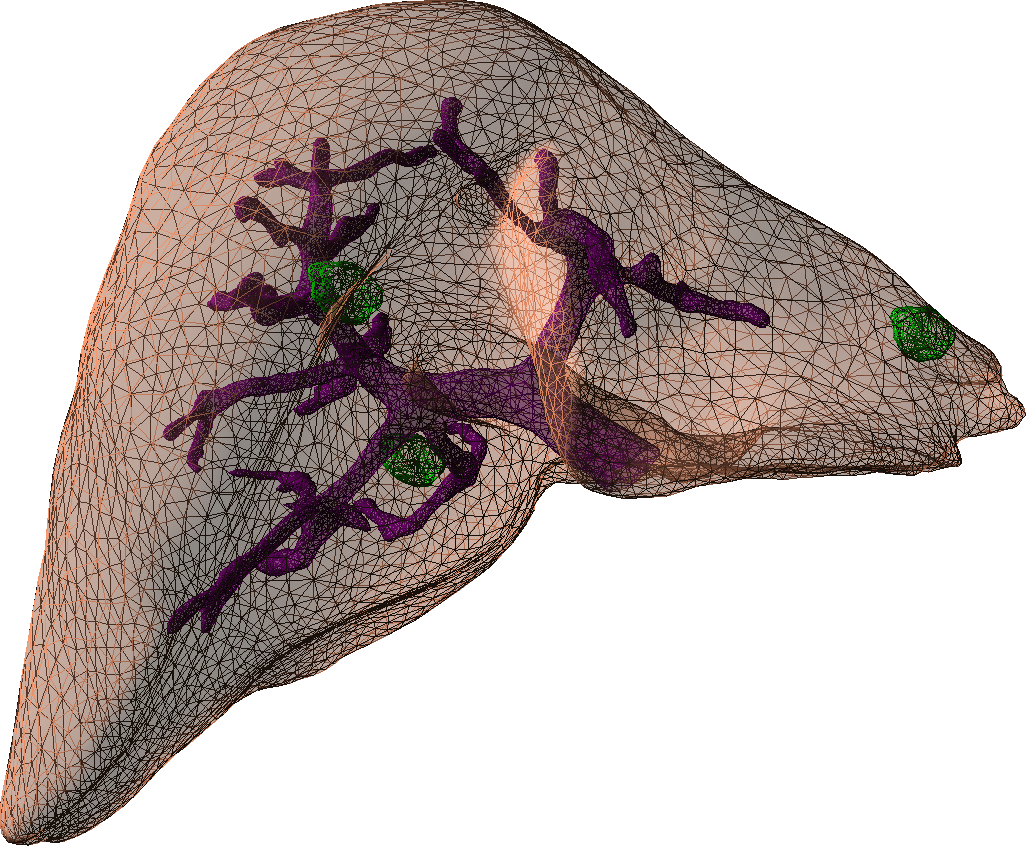
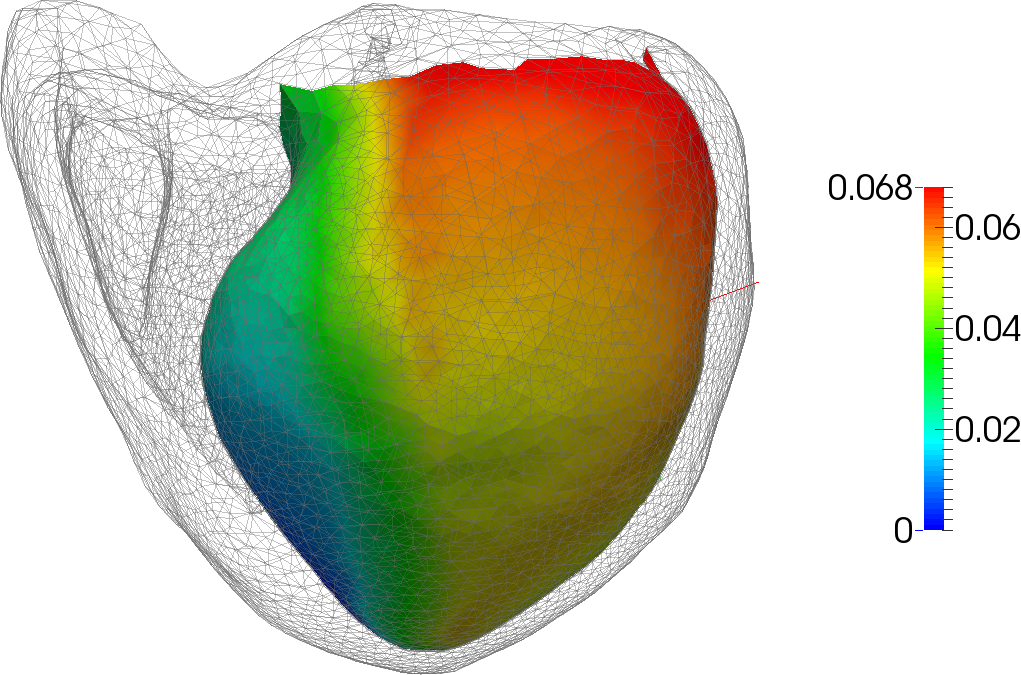
A central objective of this challenge is the modeling of the biomechanics and physiology of organs under various stimuli. This requires to describe different biophysical phenomena such as soft-tissue deformation, fluid dynamics, electrical propagation, or heat transfer. These models will help simulate the impact of different therapies (such as cryosurgery, radio-frequency ablation, surgical resection), but also represent the behavior of complex organs such as the brain, the liver or the heart (Figure 2).
A common requirement across these developments is the need for (near) real-time computation and the ability to adapt to patient-specific characteristics. Simulating such complex surgical environments involves the coupled use of composite models coming with their own discretizations that differ in terms of topology and dimension. This requires methods involving hierarchical or multi-resolution models that provide an inherent solution for the coupling of such heterogeneous representations. Another, related, objective is to study methods able to locally adapt the mesh resolution (when using an FEM approach) to the need of the simulation or to simulate the propagation of fractures during soft tissue tearing.
|
An important part of our research is dedicated to the development of new accurate models that remain compatible with real-time computation. Such advanced models not only permit to increase the realism of future training systems, but also act as a bridge toward the development of patient-specific preoperative planning as well as augmented reality tools for the operating room. Yet, patient-specific planning or per-operative guidance also requires the models to be parametrized with patient-specific biomechanical data. The objective in this area is related to the study of hyper-elastic models and their validation for a range of tissues. Preliminary work in this area has been done through two collaborations, one with the biomechanical lab in Lille (LML), and the biomechanics group from the ICube laboratory in Strasbourg on the development and validation of liver and kidney models.
Another important research topic will be related to model reduction through various approaches, such as Proper Generalized Decomposition (PGD) or modal analysis. We are currently collaborationg with the Legato team at University of Luxembourg which has good expertise in this area. Similar approaches, such as the use of Krylov spaces, have already been studied in our group recently.
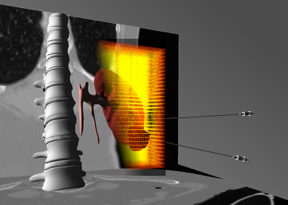
We are transitioning from our work on cardiac electro-physiology simulation to the modeling of the electrical conduction in soft tissues as well as optimization problems in the context of heat diffusion. This is a key element of the development of both planning and guidance systems for percutaneous procedures, such that an optimal therapeutical effect can be reached.