Section: Application Domains
Normal hematopoiesis
Introduction
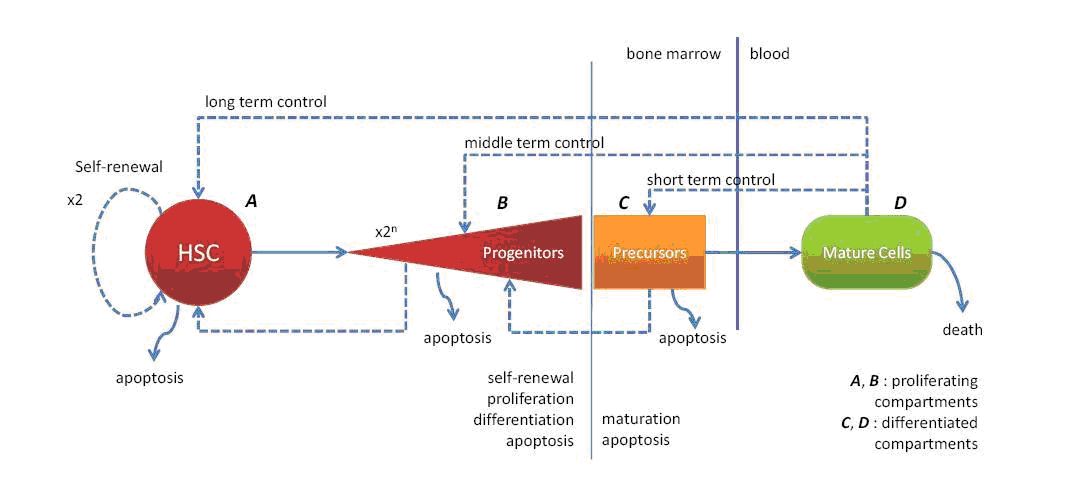
Modelling normal hematopoiesis will allow us to explore the dynamical appearance of the various cell types, originating from the stem cell compartment, through the bone marrow development up to the blood stream. The differentiated cell types will both fulfill physiological functions, and play a key role on the feedback control on homeostasis (balance of the system) in their own lineages. We will describe the hematopoiesis from three different points of view:
-
Three differentiated lineages that are responsible for specific function, namely oxygen transport, immune response and coagulation.
The basic mechanisms of our modelling approach are as follows:
-
Any cell type can have two possibilities at each time step: to divide or to die.
-
At any division step, the cell can either give rise to two daughter cells which are identical to the mother cell (self-renewal) or that are more advanced in their differentiation.
All these processes will be first modelled at the cellular level. In parallel, we will develop models of intra-cellular molecular networks (as some proteins controlling the cell cycle) influencing this decision making process, so as to be able to describe both micro-to-macro effects (molecules influencing the global cell behaviour) as well as macro-to-micro effects (like the global state of the cell population influencing the molecular behaviour).
Hematopoietic stem cells (HSC)
Although widely studied by biologists, HSC are still poorly understood and many questions remain open: How fast and how frequently do they divide? How many of them are in the bone marrow and where? How is their behaviour modified under stress conditions such as blood loss or transfusion?
Our modelling approach will be based on two methods: deterministic and stochastic differential equations with delays (discrete and distributed), on one hand, and the DPD method using the individual based modelling on the other hand. The differential equation models based on the work initiated by Mackey [42] will describe the HSC compartment in normal conditions and the behaviour of these cells under some stress. The DPD method, as a complementary approach, will emphasize the spatial regulation of stem cell behaviour, and we will focus our attention to give a possible answer regarding their location in the bone marrow and the roles of the niche, their number in the system, their possible role under stress (that is their reaction under the different feedback controls).
Blood cell functions
(i) O2 transport: red lineage
transport is provided by red blood cells (RBC) also called erythrocytes. Many different stages of maturity (including progenitors, precursors, reticulocytes and erythrocytes) are necessary to achieve the complete formation of RBC. These latter are then released in the blood stream where they transport oxygen. The whole process is tightly dependent on a robust well-balanced equilibrium called homeostasis.
It has been shown in the 1990's that apoptosis is regulated by EPO, a growth factor released by the kidneys under hypoxia. But also, under severe stress (like an important blood loss) some other molecules known as glucocorticoids can be released leading to an increase of the self-renewing rate for each generation. This led to the formulation of a first model, demonstrating the role of self-renewal.
The study of the red blood cell lineage will involve different scale levels, from the molecular one, with the effects of the hormones on the surface and internal parts of the cell, the cell contacts in each stage of RBC formation, and the red branch population in its whole with all the interactions taken into account (see Figure 3 ) in normal and stress conditions.
|
In order to couple the cellular behaviour to explicit molecular events, we will describe the events through a molecular network that is based upon the work of [46] . A first version of this model is shown in Figure 2 .
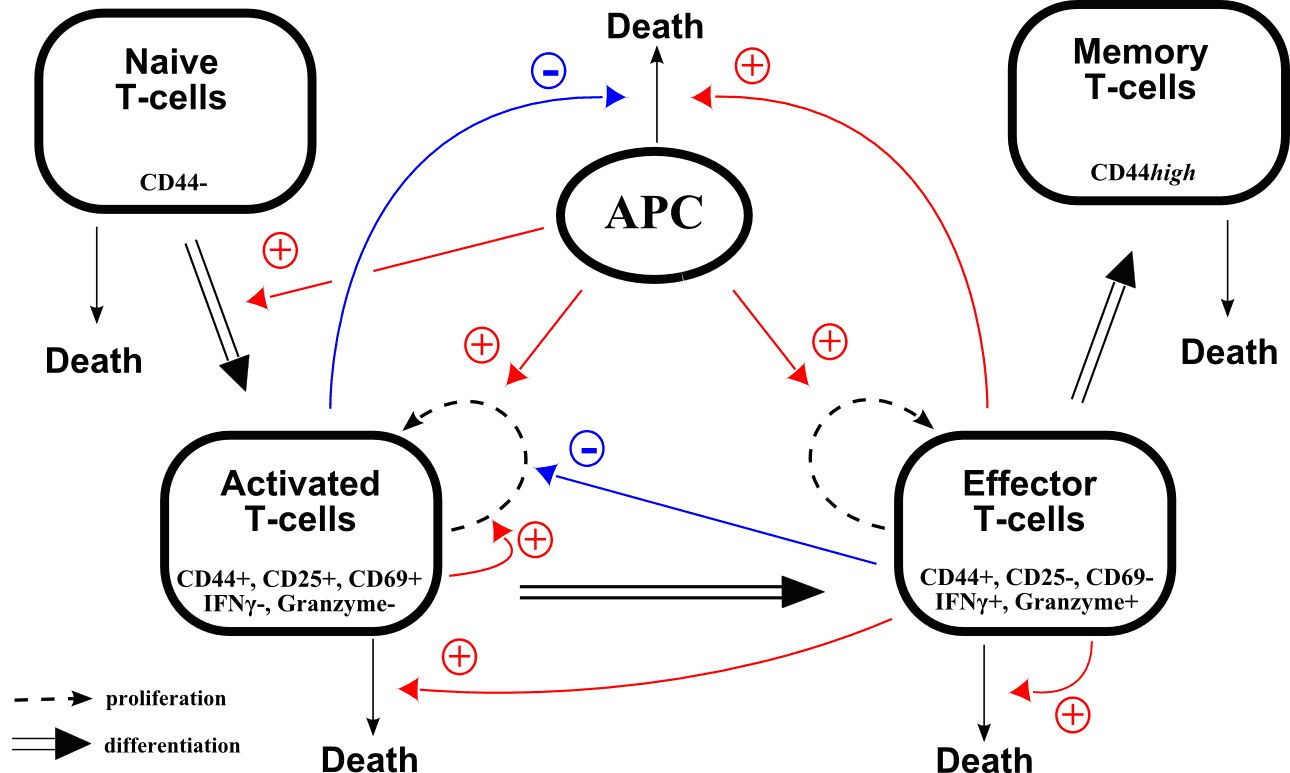
(ii) Immune response
We will focus on the production of T-cells during an immune response. This represents an important activity of the lymphoid branch, part of leucopoiesis (white blood cell production). Several models of the myeloid branch of leucopoiesis have been investigated in the frame of specific diseases (for instance cyclical neutropenia ( [41] , [38] ), chronic myelogenous leukemia [43] ).
Time evolution of T-cell counts during an infection is well known: following the antigen presentation, the number of cells quickly increases (expansion), then decreases more slowly (contraction) and stabilizes around a value higher than the initial value. Memory cells have been produced, and will allow a faster response when encountering the antigen for a second time. Mechanisms that regulate this behaviour are however not well known.
A recent collaboration just started with immunologists (J. Marvel, Ch. Arpin) from the INSERM U851 in Lyon, who provide experimental data that are essential to assess the significance of models, based on strongly nonlinear ordinary differential equations, that can be proposed for T-cell production (Figure 4 ). By considering molecular events leading to cell activation when encountering a virus, we will propose a multi-scale model of the immune response.
|
(iii) Coagulation: platelet lineage
Thrombopoiesis, the process of production and regulation of platelets, is similar to erythropoiesis although important differences are observed. These two processes have an immature progenitor (MEP) in common. Platelets are involved in blood coagulation, and can be the source of blood diseases (thrombopenia, thrombocytosis). Their production is mainly regulated by thrombopoietin (TPO), a growth factor similar to EPO.
It is important to mention that very few experimental data exist in the literature, and mathematical modelling of thrombopoiesis did not attract so much attention in the past 20 years. However, collaboration with some leading hematologists in this domain will allow us to get updated and new data regarding this process.
Deterministic models, in the form of structured transport partial differential equations, will be proposed to describe platelet dynamics, through the description of HSC, megakaryocytic progenitor and megacaryocyte (platelet precursor) compartments. Circulating TPO, regulated by platelets, will induce feedback loops in thrombopoiesis, and we will investigate the dynamics of platelet production and emergence of platelet-related diseases.